Contents
1 Aim
This standard operating procedure (SOP) represents current recommended good practice and will ensure the proper action to take regarding the setting up and management of the CME Medical T34 Syringe driver by clinical staff in the community setting.
2 Scope
This SOP is intended to be used by all community and inpatient staff within the Doncaster physical health nursing services using the CME T34 syringe driver.
3 Link to overarching policy, and or procedure
This SOP is overarched by and to be used in conjunction with safe and secure handling of medicines manual.
4 Procedure or implementation
4.1 General information
The McKinley T34 Syringe Driver is used across Rotherham, Doncaster and South Humber NHS Foundation Trust (RDaSH) in line with the requirements of the National Patient Safety Alert entitled ‘Safer Ambulatory Syringe Drivers’ (NPSA/2010/RRR019) (2010).
4.2 Safety
The McKinley T34 is a small battery operated machine, designed to give a continuous subcutaneous infusion in millilitres (ml) per hour over a given period. This method of symptom control, used predominantly in palliative and end of life care provides relief of multiple symptoms, for example, pain, nausea and agitation via a single route. The safe use of the syringe driver requires comprehensive knowledge in order to maintain patient safety.
Caution: If a patient is discharged or transferred into RDaSH services with a different make of syringe driver, this should be changed to a McKinley T34 device as soon as possible.
Lock boxes will fit syringes up to 30ml and should always be used with the CME Medical T34. Lock boxes reduce the risk of accidental or intentional interference with the syringe driver and protect from damage caused by normal daily use or drops within the range of one metre.
4.3 Decontamination and maintenance
All staff must follow standard infection prevention and control precautions including hand hygiene, use of personal protective equipment, decontamination of reusable medical devices, sharps management and disposal of healthcare waste.
The device must be decontaminated between patients, daily when in use and as and when it becomes dirty or contaminated using a 2 in 1 antimicrobial wipe. The current trust approved product is Clinell universal wipes. To prevent fluid ingress to the syringe driver it is recommended that wipes from the smaller packs of Clinell universal are used and that any excess liquid on the wipe is squeezed out before use.
Always turn the syringe driver off before decontaminating.
Also clean the actuator screw thread and guiding rods to remove debris or other particles.
Do not clean the syringe pump with chemicals such as xylene, acetone, or similar solvents. These chemicals can cause damage to components and labels.
Do not soak or immerse any part of the syringe driver in any solution. Immersing the pump in liquid will cause damage to components and this will void the guarantee.
It is recommended that Clinell universal wipes are also used to decontaminate the lock box.
All syringe drivers will must be serviced on an annual basis at the very a minimum or when a warning is indicated on the individual syringe driver’s screen. Key health solutions will provide maintenance work. Devices due for maintenance can be returned to the designated person for the clinical area, who will then forward the devices onto key health solutions. A declaration of decontamination status form will need completing and attaching to the device prior to sending it for repair or service.
4.4 Storage
It is essential that all syringe drivers are accounted for at all times.
4.4.1 Hospice
Syringe drivers and locked boxes will be stored in a designated place within the hospice. Each syringe driver will have its own case. It will be the responsibility of the nurse who last used the device to clean the syringe driver and lock box.
4.4.2 Community
All community nursing teams (planned and in planned services) are required to keep an up-to-date list of the exact location of the syringe drivers (see appendix A).
Syringe drivers will be stored at the four planned locality bases and a designated place for unplanned care. They will be stored in lockable cases and each device will have its own case. It will be the responsibility of the nurse who last used the device to clean the syringe driver.
The case will be refilled ready for further use and stored in a safe place within the base point.
4.4.3 Hospice and community
The syringe driver should be tagged as clean by wrapping the green ‘I am clean’ label around the device and storing in the lockable case.
4.5 Equipment required
- Syringe driver McKinley T34 and Lock Box. Ensure within maintenance date. Obtain a new syringe driver if out of date.
- 9-volt alkaline battery Duracell Procell. The average battery life is 3-5 days depending on usage, for example, key presses or backlight use.
- Winged infusion set Saf-T-Intima 24Gx0.75 FSP318Yellow.
- Administration set CME McKinley Micro set 100-172S (Anti-siphon valve and female Luer lock. Length approx. 100cm, priming volume approx. 0.5ml).
- BD Plastipak Luer lock syringe 20ml or 30ml. Note, Luer lock syringes must always be used to ensure secure connection of the infusion set. The pump is calibrated to Luer lock and failure to use may result in under or over infusion due to variation in syringe dimensions.
- Combi-stopper to cap the syringe or administration set.
- Transparent adhesive dressing.
- Drugs and diluent prescribed.
- Safety or blunt needle (to draw up drug).
- Drug additive label.
- Patient’s syringe driver prescription and observation chart.
- Yellow sharps bin with a yellow lid.
- Disposable carrying pouch (only if patient is mobile).
- Instruction procedure manual and equipment, as documented on syringe driver check list.
- Signing in and out sheet completed with the syringe driver serial number date, time, patient name and address (see appendix B).
- Battery must be removed when device is not in use. Syringe driver to be stored in cases provided by trust.
4.6 Hospital admission or discharge
A reciprocal agreement is in place between RDaSH and Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust (DBTHFT) for the safe return of syringe drivers to their respective bases.
The syringe driver must be returned to base after use. If the patient is admitted to hospital, then it is the responsibility of the nurse who is allocated to the visit to ensure that they retrieve the syringe driver. The responsible nurse will contact the community support manager via e-mail using the relevant form (see appendix B). An agreement is in place with DBTHFT for devices that turn up with patients that have been admitted to hospital. These devices will be returned to the medical device’s unit at DBTHFT. The medical devices unit will then return the device to the community support manager. If DBTHFT devices are sent home with patients into community RDaSH services, these will be returned by the community support manager to the medical devices unit at Doncaster Royal Infirmary (DRI).
4.6.1 Hospice admission or discharge
- If a patient is admitted to the hospice with a community syringe driver, the device must be changed to a hospice device within 24 hours and returned to the designated base via the RDaSH shuttle the next working day.
- If a patient is discharged home from the hospice with a syringe driver, the device should be swapped to a community device within 24 hours and returned to the hospice via the RDaSH shuttle the next working day.
All hospice device serial numbers are recorded on a database and tracked by the hospice ward clerk.
4.6.2 Transfer out of trust services
The registered nurse must:
- record the details and serial number of the syringe driver on the district nurse referral form or on the transfer letter if the patient is discharged to a care home, other hospital or hospice
- place a copy of the form in the patient’s notes
- request that care home, hospital or hospice return the syringe driver to medical technical services RDaSH
- inform medical technical services of the transfer
Caution, if a patient is discharged home from a neighbouring hospital (other than DBTHFT) with a different make of syringe driver, this must be changed over to the McKinley T34 as soon as possible and the other syringe driver returned to the hospital in question. The nurse changing the syringe driver should liaise with the transferring or discharge ward to arrange this.
4.6.3 Children’s Community Nursing (CCN) team
Require a prescription of drugs and diluent to be used in syringe driver.
- Need the drug or drugs name(s).
- Route to be SC.
- Duration of flow, either 12 hours or 24 hours.
- Diluent, water for injection or Normal saline.
The prescription can be in the form of a letter, medication pro forma, a CCN team referral form or a signed drug Kardex.
This letter or referral form or drug Kardex or medication pro forma is to be used as the instruction to administer and a copy MUST be kept with the appendix B in the patient home as well as in the electronic patient record.
- If a change to the dose of the medications in the syringe driver is required then a new instruction must be gained.
- Use the continuation sheet of appendix B to record the syringe driver set up and the observations.
- Record actions including batches, doses, and expiry dates within patient electronic record on SystmOne. Record in wounds and personalised care planning tab, review of patient goals and progress (same as where IV or intramuscular injection (IM) injection details are recorded).
- Ensure have allergy status recorded in SystmOne.
4.7 Administration procedure
It is good practice for two staff members, one of which must be a registered nurse, to undertake the procedure; however, in community settings where there may not be another health professional available, the patient’s relative or carer may act as a second signatory to check relevant non-clinical aspects of the procedure, for example, Stock levels.
4.8 Involving the patient and family or carers
Communication and consultation with the patient and family is essential. Before starting a subcutaneous infusion, the reasons for using this method of administration should be explained to the patient and family. Informed consent for administration should be obtained, in accordance with the consent to care and treatment policy. The discussion must then be recorded in the patient’s clinical records. If the patient is unable to give valid consent then the Mental Capacity Act should be applied as detailed in the MCA Mental Capacity Act 2005 policy. Attention should be paid to both the physical and psychological aspects involved in accepting a new way of receiving medication. Some patients feel that this method of drug delivery is a last resort and that their activities will be restricted, and that death may be imminent. It is necessary to listen to and answer the patient’s questions, acknowledge their concerns and then give explanations and reassurance as appropriate. In community settings the patient and family or carers must be informed that the syringe driver device is on loan to them, and it must be returned as delivered after use. A signature will be required from the patient or family after discussion (see appendix E).
Staff will make all reasonable attempts to reclaim equipment which is not returned (for example, following a death). If non-return of equipment is shown to be malicious (for example, re-sale), an IR1 should be completed via the Ulysses Safeguard IR1 System (staff access only) (opens in new window), and a discussion held with the Team Manager concerning reporting the incident to the police.
The explanation should include the following:
- the reasons for using a syringe driver
- how the syringe driver works
- the siting procedure and site care
- how the dose of medication can be adjusted to manage symptoms
- encouragement to describe symptoms
4.9 Procedure for setting up the syringe driver
A registered nurse must calculate the volume of drug that needs to be drawn up from the concentration of the preparation to be used and the prescribed dose. When preparing medicines and disposing of waste materials, the registered nurse must adhere to the following guidance:
- Controlled drugs (RDaSH care groups, community physical health services) SOP
- Controlled drugs (St Johns hospice) SOP
- Infection prevention and control
- Waste policy
A registered nurse must:
- ensure the prescription has been completed correctly, perform the calculation and check the medication (where the calculation is complex community staff may consider checking with a colleague before going out)
- confirm the previous opioid dose, formulation and frequency
- ensure the medication prescribed, and the doses are clinically appropriate based on the patient’s previous requirements (NPSA, 2008)
- ensure the relevant diluent has been prescribed
The diluent will be prescribed on the patient’s syringe driver prescription chart.
For McKinley T34 feature recognition please see appendix C.
4.9.1 Sterile water for injection
Advantages:
- appropriate for most medicines
- more supporting compatibility data
- reduces the risk of precipitation if using cyclizine lactate
- disadvantages
- large volumes are hypotonic and can cause infusion site pain
4.9.2 Sterile 0.9% Sodium chloride
Advantages:
- recommended with certain drugs, for example, octreotide
- less infusion site pain and fewer skin reactions than water for injection
- disadvantages
- incompatible with cyclizine
- increases likelihood of precipitation when more than one drug is used
There is a potential for interaction between drugs in a syringe driver. The compatibility of the drugs and the diluent to be drawn up must be checked prior to mixing. The Palliative Care Formulary (Wilcock et al. 2020), The Syringe Driver, Continuous subcutaneous infusions in palliative care (Dickman A and Schneider J, 2011) suggest suitable combinations of drugs.
If there are any concerns regarding compatibility:
- contact the prescriber
- contact the specialist palliative care triage
- if any further concerns out of hours (after doing all of the above) call the out of hours GP service for advice, if they are unable to answer the question the GP has access to the on-call consultant in palliative medicine via DRI switchboard
Ideally syringe driver prescription reviews should take place in working hours only (8:30am to 4:30pm, 7 days per week).
Routinely in RDaSH a combination of no more than 3 drugs should be prescribed on the prescription form or drawn up into a syringe.
- Expected process, if more than 3 drugs are required, 2 prescription forms and 2 syringe drivers will be needed.
- Exceptionally 4 drug combinations may be appropriate on an individualised basis only, the rationale and compatibility must be clearly documented in the patient’s clinical record.
Please seek advice from the Specialist Palliative Care team.
Note, ampoules containing a liquid solution may contain an amount in excess of the volume stated on the label. Therefore, measure the volume of liquid as it is drawn up to ensure accuracy of the dose.
- Select the appropriate syringe size 20ml or 30ml.
- Draw up the prescribed medication.
- Add the diluent up to the required volume.
- Complete the label, including:
- patient name and NHS number
- medicine name and dose
- batch numbers
- diluent used
- route of infusion
- date and time prepared
- initials of the registered nurses preparing the syringe
- Attach the label to the syringe ensuring that it is flat and not folded. Leave the scale visible so that it can still be read.
- Skin site selection for subcutaneous infusions. Where possible involve the patient in the choice of a suitable site. Areas suitable for subcutaneous infusion include those with a good depth of subcutaneous fat, towards the trunk of the body, particularly if the patient’s peripheral circulation is compromised.
Following completion of the procedure, document the following in the patient’s electronic records:
- the prescribed medication
- dose
- diluent
- site of the syringe driver
- alongside the amount of prescribed medication remaining (see appendix B)
Note when using appendix B:
The CME Medical T34 ambulatory syringe pump is calibrated to operate with BD Plastipak Luer lock syringe brands and sizes. It is programmed to recognise both the brand and the size of syringe being used (commonly 20ml and 30ml). It will be defaulted using a prime and load (lock, on) programme of a 24-hour delivery system.
4.9.3 Fill volume
Medication and diluents should always be drawn up to the following volumes:
- 20ml syringe draw up to 17ml
- 30ml syringe draw up to 22ml
4.9.4 Acceptable sites
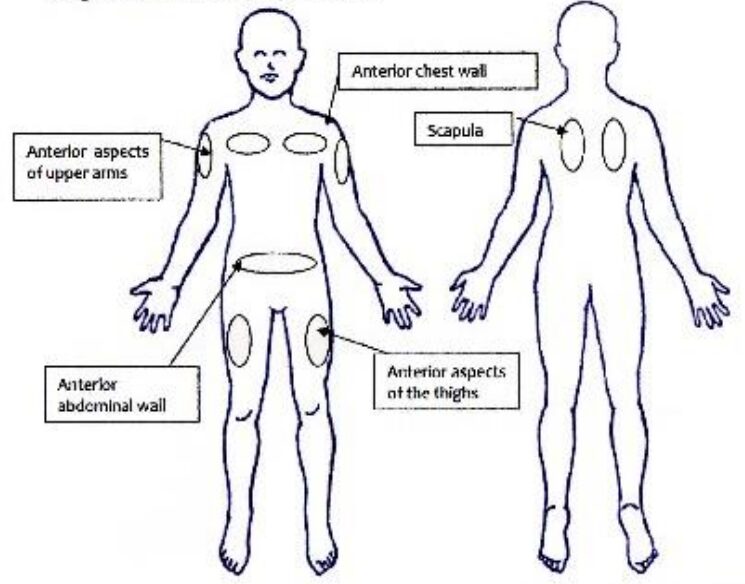
Subcutaneous injection sites acceptable sites:
- anterior aspects of upper arms
- anterior chest wall
- anterior aspects of the thighs
- anterior abdominal wall
- scapula
4.9.5 Sites to avoid
- Oedematous areas, including areas affected by lymphedema or ascites (poor absorption, increased risk of infection).
- Sites over bony prominences (discomfort and poor absorption).
- Joints or skin folds (discomfort and movement may displace cannula).
- Previously irradiated skin (may have poor perfusion, affects drug absorption).
- Upper abdomen in a patient with an enlarged liver (risk of puncturing the liver capsule).
- Upper chest wall in a very cachectic patient (risk of pneumothorax).
- Infected, broken, inflamed or bruised skin.
- Sites of tumour.
4.9.6 Inserting BD Saf-T-Intima
Decontaminate hands using alcohol rub or soap and water or for community settings soapy hand wipes can be used followed by alcohol hand gel.
- Clean the site with a 2% chlorhexidine/70% alcohol skin preparation wipe and allow to dry for 30 seconds.
- Apply the clamp to the line.
- Grasp the “pebbled” side of the wings of the cannula, pinching the wings firmly together. This “locks” the needle and prevents it from retracting during insertion.
- Ensure that the needle is point down and bevel uppermost, to guide the cannula through the tissues. This prevents kinking of the cannula. If the needle is not already orientated with the point down open the wings and gently twist the white shield until the needle is correctly positioned.
- Insert the cannula subcutaneously at an angle of less than 45 degrees, lowering the initial angle of the cannula to a level more parallel to the skin (caution on the chest wall) and advance to the hilt of the cannula.
- Open the wings (pebbled side down) flat against the skin.
- Apply transparent adhesive dressing over the insertion site and the cannula wings.
- Apply firm finger, tip pressure over the wings of the cannula (avoiding the centre where the needle retracts) simultaneously grasp the pebbled end of the coloured or white cylinder shield and pull in a straight continuous motion until the needle has fully with drawn into the cylinder and pops off.
- Gently remove cylinder from the cannula port, if it has not released spontaneously, exposing the adapter with the rubber bung.
- Place the needle shield in the sharps bin.
- Decontaminate hands.
- Change the Saf-T-Intima and site every six days or as necessary depending on the condition of the site.
- Change the infusion line every three days as a minimum or at dose change.
- Exceptionally, where a patient is in the last hours of life consideration should be given to the disruption of the patient against the clinical benefit from changing the Saf-T-Intima.
Note, the Saf-T-Intima cannot be primed when using the McKinley T34. When setting up the syringe driver the primed extension line is connected directly to the un-primed Saf-T-Intima.
The registered nurse must prepare the syringe and confirm the patient’s identity as on prescription:
- check the battery life by pressing the “INFO” key twice
- prepare syringe and contents and cap the syringe
- press “STOP”
- press and hold “INFO” key to deactivate keypad lock
- if the infusion complete alarm has activated, press “YES” to confirm end of infusion
- switch syringe driver “OFF”
- clamp Saf-T-Intima
- raise the barrel clamp arm
- remove old syringe
- follow instructions on page 18 power on and pre-loading
- when “start infusion?” screen displays, connect the infusion line to the syringe, release the clamp on the Saf-T-Intima and press “YES”
In community settings the battery must be changed when the device registers at 40% battery life left.
4.9.7 Bolus dose of medication
If the patient has uncontrolled symptoms prior to setting up the McKinley T34, it may be necessary to give a PRN dose of medication.
A separate Saf-T-Intima 24Gx0.75 FSP318 Yellow should be inserted for the purpose of giving PRN doses. A Swan Lock needle free device should be attached to the Saf-T-Intima port.
Administer medication slowly (30 seconds to 1 min) dependent on volume and comfort. If using more than one drug:
- ensure they are compatible. If not compatible use a separate Saf-T-Intima device and label it per PRN medication
- do not mix the medications in one syringe
- do not use a pre-flush at the start
- flush with 0.3mls saline between medications and always flush at the end
- no more than 2mls (including flush) to be administered in one episode (per device), it is normal to have a small swelling immediately after administration of medication (advise the patient and family of this to alleviate anxiety)
4.9.8 Temporary interruption to the infusion, hospice
This is not normal practice in a community setting and should only be used in exceptional circumstances, for example, showering or bathing.
Note, stopping the infusion will delay the end time of the infusion.
4.9.9 Stopping the infusion
- Press STOP, unlock the keypad and turn the syringe driver OFF.
- Clamp the Saf-T-Intima.
- Disconnect the syringe driver. Do not remove the syringe from the pump.
- Cap the Saf-T-Intima and infusion line to minimize cross infection.
- Record time and reason why infusion stopped on syringe driver observation chart.
4.9.10 Resuming the infusion
- Check that the prescription, syringe label and patient details are correct.
- Reconnect the line to the syringe on the pump maintaining asepsis.
- Press and hold ON button.
- The screen will request confirmation of size and brand of syringe.
- Press YES to confirm.
- The screen will display “Press YES to Resume” “NO for New Syringe”.
- Press YES to resume.
- The screen will display remaining volume or duration or rate of infusion.
- Ensure information is correct as prescribed.
- Press YES to confirm.
- Unclamp Saf-T-Intima.
- Screen will display “Start Infusion?”.
- Press YES to start infusion.
- Press and hold INFO key to lock the keypad.
- Replace the Lock Box.
- Record on the syringe driver observation chart the time the infusion was recommenced.
Note, if “NO for New Syringe” is pressed the remaining infusion will be reset to deliver over the next 24hrs at a different rate, and the infusion will be incorrect.
If “NO for New Syringe” has been pressed in error. Discard the remainder of the syringe contents, prepare, and set up a new syringe. In a community setting the following procedure should not be applicable as staff will check the battery status before commencing each 24-hour regime.
4.9.11 Changing the battery during an infusion
- When near the end of the battery alert sounds, it indicates that the battery should be changed within the next half hour.
- Press STOP and apply clamp to Saf-T-Intima.
- Insert new battery.
- Restart the syringe driver.
- Reconfirm syringe size and brand.
- Press YES to resume.
- Reconfirm volume and duration and rate of infusion.
- Press YES to recommence the infusion.
- Lock the keypad.
Following completion of the procedure all patient records must be updated and signed by the registered nurse. (For paper documentation see appendix B). Records will also include those accessed on SystmOne.
4.9.12 What to do if there is an occlusion in the extension line
- Look at the syringe driver screen for any error messages and, if possible, resolve as required.
- Check that the Saf-T-Intima is not clamped.
- Check the extension line and Saf-T-Intima for any kinking, straighten if kinked.
- Check the syringe, extension line and Saf-T-Intima for any signs of crystallization of the solution. If crystallization detected, prepare and fit new syringe, extension line and change Saf-T-Intima site.
- If no physical reason for the occlusion is detected, resite the Saf-T-Intima.
- If occlusion is still present, change the site, extension line and syringe.
Note, during an occlusion the pump’s post occlusion reduction system will reverse the operation of the motor and drive the actuator backwards, otherwise the pressure build up could cause a surge of fluid into the patient on release of the occlusion. When the pump is resumed or restarted following the backward movement of the actuator, time will be added to the time remaining to protect the original calculated rate.
4.9.13 Procedure for releasing a trapped foreign object from the actuator (see appendix D)
Warning, if a foreign object is trapped in front of or behind the actuator during pre-loading (automatic actuator movement) or when manually adjusting the actuator, the user should:
- Ignore screen prompts as the prompt that may display will be in relation to alarm activation and NOT the trapped object.
- Turn the syringe driver OFF.
- Raise the barrel clamp arm and turn it to the left or right to keep it in the raised position.
- Turn the syringe driver ON.
- Turn and lower the barrel clamp arm.
- Use the FF key (or BACK key) to move the actuator in order to release the object.
4.10 Faulty device
If the syringe driver is faulty, it needs to be changed for a non-faulty device as above, an IR1 may need completing is treatment delayed – seek advice from line manager. The faulty device must be cleaned and labelled as cleaned using the green ‘I am clean’ label. The designated syringe driver coordinator for each area, for example, in the hospice it is the ward clerk, registers the device out of action and sends to the trust’s designated repair contractor.
4.11 Incident reporting
Systems are in place within RDaSH to report and manage incidents, near misses and serious untoward incidents involving syringe pumps. Staff should be familiar with the reporting process and the Incident management policy.
4.11.1 Acknowledgements
Illustrations courtesy of:
5 Training
All registered nurses using the CME T34 Syringe Driver must be personally competent and accountable in the use and operation of the device by attending bespoke training. Evidence of training will be recorded on each staff member’s training matrix; annual updates will be provided. The training could be completed on a 1-to-1 basis using care and clinical skills assessment tool (CCAST) assessment. An assessor will accompany the registered practitioner and complete the CCAST documentation alongside some medicine management training around calculations or drugs and so on. The results will then be recorded on the staff member’s training matrix.
6 Links to associated documents
7 References and literature review
- Dickman, A, and Schneider, J 2011. The Syringe Driver. Continuous subcutaneous infusions in palliative care 3rd ed. Oxford: University Press Oxford.
- Medicines Act 1986. HMSO: London Mental Capacity Act (2005) DOH: London National Patient Safety Agency 2010. Rapid Response Report NPSA/2010/RRR019: Safer ambulatory syringe drivers.
- National Patient Safety Agency.
- Wilcox, A. Howard, P and Charlesworth, S (2020) Palliative care Formulary, 7th ed, Nottingham, pharmaceutical press.
7.1 Bibliography
- Mitten, T. (2001) Subcutaneous drug infusions: a review of problems and solutions.
- NMC (2015) The Code: Standards of conduct, performance and ethics for nurses and midwives, Nursing and Midwifery council, London.
- Perdue, C. (2004) The syringe driver, an aid to delivering symptom control. Nursing Times, 100, 13. pp32 to 35.
- Wilson J. (2019) Infection Control in Clinical Practice. Updated Third Edition. Elsevier, London.
8 Appendices
8.1 Appendix A Syringe drive base information
8.2 Appendix B Subcutaneous syringe driver instruction and observation chart for McKinley T34
8.3 Appendix C McKinley T34 feature recognition

- Barrel clamp arm secures syringe and detects brand and size.
- Collar sensor detects correct loading of syringe collar.
- Plunger sensor detects correct loading of the syringe plunger.
Actuator:
- drives the syringe plunger to deliver the syringe contents
Info key:
- shows infusion summary, protocol parameters, battery level
- when pump is paused, access the main menu
- activates or deactivates keypad lock UP arrow
key:
- scrolls between options
- increases infusion parameters Down arrow
key:
- scrolls between options
- decreases infusion parameters during programming or titration
Yes or Start:
- confirms selection and starts infusion
No or Stop:
- stops infusion
- takes the user back a step during programming
FF (forward):
- moves actuator forward when no syringe in place and barrel clamp arm is down
- accesses purge function (if enabled)
Back (reverse):
- moves actuator backward when no syringe is in place and barrel clamp arm is down
On or Off-key:
- switches the pump on and off infusion LED light
- a green indicator lights:
- during system self-test
- intermittently to indicate infusion delivery
- a red indicator light:
- continuously to indicate an alarm state
- when pump paused or on stand-by mode
8.4 Appendix D Procedure for released a trapped, foreign object from actuator
8.5 Appendix E Loan form
Document control
- Version: 5.1.
- Unique reference number: 150.
- Approved by: Clinical policies review and approval group.
- Date approved: 3 October 2023.
- Name of originator or author: Chief pharmacist.
- Name of responsible individual: Executive medical director.
- Date issued: 27 October 2023.
- Review date: April 2025.
- Target audience: Physical health nursing staff.
- Description of change: The addition of information specific to the management of children and young people by the children’s community nursing team section 4.6 added.
Page last reviewed: February 25, 2025
Next review due: February 25, 2026
Problem with this page?
Please tell us about any problems you have found with this web page.
Report a problem