Contents
- Introduction
- Clozapine clinics
- Patient procedure
- Activating procedure for point of care analysers (PocHi)
- Internal quality control (QC procedure)
- Analysing blood samples
- If patient’s results is not accepted by ZTAS
- Patient’s blood samples
- Quality assurance
- PocHi problems
- Regular maintenance and stock
- Medications
- End of clinic
- Other information
- Appendices
1 Introduction
The trust has welcomed the introduction of a point of care haematology (PocHi) analyser into Clozapine clinic which is located at the Ironstone Centre, Scunthorpe.
Clozapine is an atypical antipsychotic that requires to be validated full blood counts (FBCs) to be available prior to dispensing of medication to service users. The introduction of the point of care (PocHi) blood testing systems will be beneficial to service users in that the opportunity exists for the collection of Clozapine medication on the same day as venepuncture because blood sample results are available within 5 minutes.
The procedural information detailed in this document is one of a suite of documents to provide guidance on the governance of point of care analysers to Rotherham, Doncaster and South Humber NHS Foundation Trust.
2 Clozapine clinics
Clozapine clinics have developed to monitor patients who are prescribed Clozapine. They monitor patients who require weekly, fortnightly, or monthly blood tests, review their mental state and monitoring of their vital signs, including ECG (yearly).
The clinic is led by the Lithium or Clozapine clinic nurse coordinator who provides and coordinates care in collaboration with the patient’s RMO, care coordinator or lead professional and ward, as appropriate.
With the introduction of the point of care analyser, the Clozapine
Clinic nurse and the nursing assistant have been trained to operate the analysers in accordance with stringent operating procedures. Each clinic has a named operator who is accountable for safe operation of the analyser and to ensure that standards are maintained.
3 Patient procedure
Patient attends Clozapine clinic as per appointment.
The clinic nurse will review the patient’s mental and physical state and a routine blood sample will be taken.
Patients may be asked to stay until their blood results are available to enable medication to be dispensed following a ‘green’ blood result.
4 Activating procedure for point of care analysers (PocHi)
The operator of the PocHi analyser must have undertaken PocHi training and be a certified (trained by Sysmex) or registered (trained by a trust trainer) operator.
The operator must follow the trust’s infection control procedures.
At the beginning of the day, immediately upon switching on the PocHi machine, a background check runs automatically. After this the quality control sample must be run (see section 5). A satisfactory (or valid) quality control test is required prior to the testing of patient blood samples with the PocHi. The entire start-up procedure should take a total of thirteen minutes.
5 Internal quality control (QC procedure)
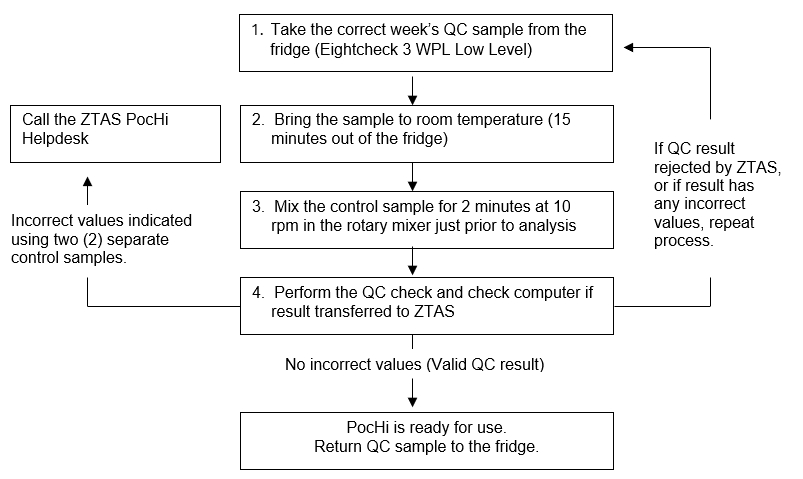
- Take the correct week’s QC sample from the fridge (Eightcheck 3 WPL low level).
- Bring the sample to room temperature (15 minutes out of the fridge).
- Mix the control sample for 2 minutes at 10 rpm in the rotary mixer just prior to analysis.
- Perform the QC check and check computer if result transferred to ZTAS:
- no incorrect values (valid QC result), PocHi is ready for use, return QC sample to the fridge
- incorrect values indicated using two (2) separate control samples, then call the ZTAS PocHi helpdesk
- if QC result rejected by ZTAS or if result has any incorrect values, repeat process, proceed back to step 1 and repeat process
If the QC result is within the limits of the QC target result, the computer subsequently displays the following messages:
- “Quality Check successfully completed”
- “PocHi 100i ready for use”
If the QC result is outside the limits of the QC target result:
- the PocHi indicates the incorrect values on the PocHi display and the printout
- the computer subsequently displays the following messages:
- “QC failed: result does not match reference values”
- “Please perform Quality Check.
Repeat steps 1 to 4 above.
If on a second attempt the result from the QC tests fails:
- the computer subsequently displays the following messages:
- “QC failed (2): result does not match reference values”
- “PocHi has been disabled, please contact ZTAS.”
- call the ZTAS helpdesk on 0207 365 58 42
In case you have failed 2 consecutive QC tests, ZTAS and Sysmex will automatically be informed. You will however need to contact ZTAS to initiate the process of problem-solving with your PocHi. The cause of your repeated QC failure must be investigated. If necessary, ZTAS will consult Sysmex for advice to solve the problem. Once the problem is solved, ZTAS will re-set the PocHi software for a repeat QC analysis, if applicable.
Possible reasons for QC failure:
- commonly, the sample has not been warmed or mixed sufficiently
- less commonly:
- PocHi fails, PocHi needs repair or replacement
- Sample corrupted, repeat the QC with another sample
- QC lot expired
- QC lot not recognised
- internet communication failure
All QC check results should be recorded in the Sysmex PocHi analyser daily log sheet (appendix B).
6 Analysing blood samples
Blood samples must be labelled with a ZTAS sticker and analysed as soon as possible after they are taken, always within 8 hours (samples must be kept in the refrigerator if it is expected that analysis will be more than 4 hours from sampling, note, bring to room temperature before testing).
- Insert a valid ZTAS user ID in the car reader and log on (if applicable). The computer should display the message “PocHi-100i ready for use”.
- On the PocHi, select “WB” (whole blood) analysis mode.
- Scan the patient’s ZTAS number using barcode scanner.
- Ensure that the computer the PocHi software is connected to the internet and the PocHi PC software is running.
- Mix blood sample for two (2) minutes on the rotary mixer at 10 rpm and put into the PocHi.
- Visually confirm the personal identification number (PIN) number match on the screen and the sample.
- Press “Run” on the screen, analysis takes approximately 2 minutes.
- The result is automatically transferred to ZTAS and is visible on the PocHi display.
- If the patient result has been accepted by ZTAS, the computer displays the message, “Result transferred to ZTAS database”. The result is automatically submitted to the ZTAS database and the ZTAS website interprets and classifies the result instantly:
- amber result:
- ZTAS will start the alert procedure
- refer to Clozapine dispensing protocol
- green result:
- refer to Clozapine dispensing protocol
- red result:
- contact ZTAS commence red result procedure, refer sample to local laboratory for re-test, ensure medication is removed from patient
- amber result:
7 If patient’s results is not accepted by ZTAS
The following are possible reasons:
- ZTAS PIN of the patient is not recognised:
- invalid patient identifier used.
- patient not registered with ZTAS.
- a second result for the same patient is submitted on the same day
- the sample has an invalid particle distribution:
- the sample is not sufficiently mixed
- the sample has clotted
- there is no sample tube in the PocHi during analysis, or the sample tube is empty or filled with watery fluid, values outside acceptable ranges.
- internet communication failure
8 Patient’s blood samples
Following analysis of the sample, it is stored in a specimen fridge. All samples from the weeks clinics are kept until the following Monday.
Samples tested on two occasions that have failed due to an invalid result “invalid particle distribution” should be sent to the local lab for analysis, or second sample can be taken and run on PocHi.
Used or analysed blood samples or tubes are disposed of in plastic orange-lidded burn bins as advised.
9 Quality assurance
The type of quality control (QC) blood samples used for the internal quality control (see sections 4.0 and 5.0) of PocHi is Eightcheck 3 WPL (low level).
ZTAS has a standing order with Sysmex for Rotherham, Doncaster and South Humber NHS Foundation Trust for delivery of new QC samples every 3 months.
Every 3 months, a new pack of QC samples are delivered to PocHi clinic sites. These will be delivered to the Ironstone Centre and the clinic coordinator informed of arrival.
The pack of QC samples must immediately be stored in the sample fridge. Upon receipt the delivery must be checked to ensure the correct type of QC samples ‘Eightcheck 3 WPL (low level)’ red tops and within date have been received.
If no new QC samples have been received a week before the expiry date of the batch in use, the ZTAS Helpdesk must be contacted.
The QC samples are stored in a locked fridge at a temperature of between 2 and 8 degrees centigrade.
The new pack of QC samples must only be used once the previous pack has expired.
Prior to the use of the new QC pack, the batch number, expiry date and reference values should be entered into the PocHi (using barcode scanner).
A QC sample that is (to be) used should have two dates inserted on the tube label, one date when the sample is first used and the second date being the discard date one week later.
9.2 PocHi external quality assurance (NEQAS)
ZTAS have registered your PocHi with the national external quality assurance scheme.
You are required to participate in the external quality control on a monthly basis in order to keep your PocHi service operational.
At the beginning of each month, a set of NEQAS samples is delivered to PocHi clinic sites. These will be delivered to the Ironstone Centre and the clinic coordinator informed of arrival.
The NEQAS samples must be tested, as if they were patient samples, and the result returned to NEQAS within the indicated timeframe on the enclosed instruction sheet.
The NEQAS samples must be kept in the sample fridge until they are being tested.
Instructions for testing are available with each set of NEQAS samples.
If no new NEQAS samples have been received by the second half of the month, the ZTAS Helpdesk must be contacted.
9.3 Record keeping or file maintenance
Keep temperature log, every day your PocHi is in operation, check the temperature of the fridge that stores QC samples (see section 9.1) to confirm that the samples are appropriately kept. Record or make a note on your temperature log (date and signature).
Maintain daily log sheet (appendix B), with relevant information on each day that the PocHi is in operation.
Training records, keep training records for trained operators.
10 PocHi problems
10.1 PocHi failure
In the event of PocHi failure, ZTAS must be informed.
If the problem cannot be solved by ZTAS, the patients’ blood samples will be tested by Doncaster and Bassetlaw NHS Foundation Trust.
ZTAS will ensure that the PocHi is repaired or replaced within 3 working days, usually the next working day.
10.2 PocHi software internet communication failure
When the PocHi software or the internet communication fails, the PocHi results will not be transferred to ZTAS. ZTAS must be contacted.
If the QC check has already been performed that day, and the result is valid (accepted by ZTAS), the PocHi can still be used for analysis of patients’ samples.
If the QC has not been performed for that day, ZTAS must be informed for resolution of the problem. If the problem cannot be resolved the patients’ blood samples will be tested in the local lab.
ZTAS will attempt to ensure that the problems with the PocHi software are solved within 3 working days, usually by the next working day in liaison with trust’s IT department where necessary.
In the event of temporary internet communication failure, the PocHi software will continue to transfer the last result to ZTAS within the next 3 hours. This result will be transferred to ZTAS upon restoring the internet communication. The 100 last results are stored in the PocHi. If necessary or applicable the stored results can be submitted to ZTAS through the ZTAS PocHi software.
In the event that ZTAS is not available, the PocHi result should be compared with reference values as stated in the ZTAS manual and discussed with the dispensing pharmacy before Zaponex is dispensed. Results must be submitted to ZTAS later on (keep paper copy of ZTAS manual on hand for emergency)
11 Regular maintenance and stock
11.1 PocHi reagents and fluids
A stock of PocHi pack 65 and Cell Clean, the cleaning agent, are kept in a locked cupboard at all times in the Clozapine Clinic at ambient temperature.
To re-order Cell Clean, PocHi pack 65, contact the ZTAS Helpdesk via email at infor@ztas.co.uk.
Re-Ordering is performed as the stock level decreases to a (recommended, one weeks’ supply) of PocHi pack 65 and Cell Clean, 1 bottle.
Protective goggles, gloves and plastic aprons should be worn when changing and handling reagents. All spillages will be cleaned according to safety sheet (see attached document).
The PocHi pack 65 reagents will be disposed of by adding the Vernacare granules to solidify the liquid then taping up the box. The box can then be double bagged in an orange waste bag.
After rinsing out, the cell clean bottle can be either put into the plastic recycling or general household waste.
11.2 Changing reagents
- The PocHi prompts that reagents need changing or change is deemed necessary by the operator:
- PocHi prompt, press “Execute”
- operator decision, press “Menu” and select “Chg Reag”
- Scan in or manually enter the Lot#, expiry date and serial number of the new pack.
- Replace the pack to be changed.
11.3 Transducer cleaning, prompted automatically
Performed every 2 weeks or after 150 blood samples.
- Add 1ml of cell clean to an empty sample tube.
- Put the tube, without cap, in position in the machine:
- if PocHi prompts to clean press “Execute”, the process will take approximately 9 minutes.
- If not cleaning in response to PocHi machine prompt, then press “Maint”.
- Press execute, the process will take approximately 9 minutes.
11.4 Waste chamber cleaning, prompted automatically
Performed every 3 months or after 1500 samples.
- Add 1ml of Cell Clean to an empty sample tube.
- Put the tube, without cap, in position in the machine:
- if PocHi prompts to clean then press “Execute”, the process will take approximately 13 minutes
- Select “Clean W. Chamber”.
- Press “Execute”, the process will take approximately 9 minutes.
12 Medications
Medication will be delivered from Lloyds pharmacy every Friday morning.
Medication is to be checked against the Clozapine clinic log sheet and checked that each patient’s medication is present, and the log sheet signed accordingly.
Medication is then to be stored in the drug cupboard (key is locked in the key cupboard located in the reception office, access by qualified nurse or nominated pharmacy team)
Following a ‘green’ result medication is to be handed to the patient and recorded accordingly on the clinic log sheet.
When clinics are finished the clinic log sheet is to be emailed to Lloyds Pharmacy (mhtbarnsleyinterchange@nhs.net) for their records.
Original clinic log sheet to be filed in correct folder.
13 End of clinic
Ensure at the end of the clinic that:
- computer is shut down correctly
- POCH-100i machine is shut down correctly
- medication cupboard is locked and key stored back in the key cabinet
- treatment room door is locked to restrict access
14 Other information
This operating procedure is to be used in conjunction with the protocols on red and amber blood results and local blood testing.
15 Appendices
15.1 Appendix A PocHi operators and clinics sheet
15.1 Appendix B Sysmex PocHi analyser daily log sheet
Page last reviewed: October 14, 2024
Next review due: October 14, 2025
Problem with this page?
Please tell us about any problems you have found with this web page.