Contents
1 Policy summary
This policy is designed to provide guidance for the practice of non-medical prescribing (NMP) in Rotherham, Doncaster and South Humber NHS Foundation Trust RDaSH) (the trust). The policy details what conditions must be in place before practitioners may prescribe within the legal NMP framework, their agreed formulary and job role within the trust. The policy includes details of the processes to follow and includes the forms within the appendices, which are required to be completed at relevant times, to provide a governance framework and ensure safe practice.
2 Introduction
Non-medical prescribing is utilised across the trust by practitioners who have undertaken specific additional training and been examined against core competencies. Practitioners work only within roles that have been identified as being appropriate for non-medical prescribing, to support the patient experience and journey.
This policy outlines governance arrangements to promote safe and effective practice and to provide assurance to the trust around non-medical prescribing practice.
This policy details actions that may be taken to suspend or terminate trust authorisation for an NMP to prescribe.
3 Purpose
The purpose of this policy is to set out the legal framework, standards, academic, experiential, and procedural requirements to facilitate a safe, effective and a clinically valid framework for non-medical prescribing practice to take place within the trust.
It will detail which practitioners can supervise non-medical prescribers (NMPs) and what criteria must exist before those practitioners undertake supervision of NMPs.
4 Scope
This policy is applicable to all non-medical prescribing clinicians (colleagues), supervising practitioners, matrons, service managers, care group nurse directors, head NMP, care group NMP leads working across all trust clinical services and locations where there are NMP’s within the service.
For further information about responsibilities, accountabilities and duties of all employees, please see appendix T.
5 Procedure or implementation
5.1 Quick guide
5.1.1 Types of prescribers
- There are three types of non-medical prescriber within the trust, which are determined within practitioner roles and responsibilities, they are:
- supplementary prescriber
- independent prescriber
- community practitioner nurse prescriber
5.1.2 Training
- Requests for non-medical prescribing training need to go through the approved routes as detailed in the policy.
- A designated prescribing practitioner must be identified before applying for the course.
- The colleague is responsible for completing the paperwork and arranging to meet with the care group NMP lead for approval.
- Approval must be sought and agreed before applying to the university.
5.1.3 Gaining authorisation
- Once the NMP qualification has been gained, approval must be sort within the trust before commencing prescribing.
- The process for this is detailed in the policy.
- Seeking changes to smart card rights, registration at prescription pricing authority (PPA) and on SystmOne.
5.1.4 Record keeping
- Complete records as soon as possible after an event.
- If there is no time to make a full entry, record the pertinent information including medication changes.
- There are specific requirements for supplementary prescribing which are detailed in the policy.
5.1.5 Clinical governance, evaluation and audit
- NMP’s must have a supervisor and access regular supervision (minimum of every 90 days) that supports their prescribing practice.
- The Competency Framework for all prescribers will be used as a tool within clinical supervision and for sharing good practice.
- An annual declaration will be completed each year in April to cover the previous 12 months prescribing practice within the trust.
5.1.6 Probity and ethical issues
- All NMPs must prescribe within their registration body’s standards for prescribing practice.
- Under no circumstances can an NMP accept free samples.
- NMPs are accountable for their practice at all times. If circumstances arise where an NMP is using their prescribing qualification in private practice, for example, aesthetic procedures then they must declare this on their annual declaration.
- Where a patient is unable to give their consent to the supplementary prescribing agreement to the NMP arrangements, the NMP cannot proceed.
5.2 Selection criteria for non-medical prescribing training
The selection of individuals for non-medical prescribing training must be dependent on the role they will undertake. The NMP University course application process is shown at appendix A. For practitioners undertaking the community practitioner nurse prescribing qualification as a part of the health visitor or school nursing training, this is a component of the application for that training course which includes the NMP module and as such does not go through the training request format detailed within this policy. The advanced clinical practitioner training also includes prescribing as a core module that does not need to seek the additional approval.
The following criteria will be applied to all supplementary and independent NMPs:
- first level professional qualification
- confirmed named designated prescribing practitioner (DPP) Supervisor or agreed NMP supervisor
- agreed support from care group NMP lead to attend course (the NMP lead will link with management colleagues and, or nurse director as appropriate to agree on suitability)
- ability to study at a minimum of level 6
- registration with a national professional body, Nursing and Midwifery Council (NMC), General Pharmaceutical Council (GPhC), Health and Care professions Council (HCPC)
Following qualification, NMPs must have access to:
- named NMP supervisor
- patient records
- a formulary of drugs to prescribe which has been agreed with their supervisor
- prescription pad or inpatient drug card or computerised prescribing system access
- pharmacist advice
- protected continuous professional development time per year for updating on relevant prescribing issues, for example, reading of journals, attending supervision
- clinical supervision related to prescribing at least every 90 days (recorded on portal)
- peer NMP support
Should service needs change at the point of an individual’s successful completion of the NMP University course and they are not required to be an active NMP, then it is the responsibility of the appropriate line or service manager to discuss the NMP status with the individual direct and inform the NMP lead in their care group.
5.2.1 Supplementary prescribing
- Supplementary prescribing is a voluntary partnership between a doctor or dentist and a supplementary prescriber to implement an agreed patient specific clinical management plan (CMP) with the patient’s agreement.
- A supplementary prescriber may currently be a specially trained nurse or pharmacist who can prescribe any medicine within their clinical competence.
- According to a patient specific CMP (see appendix K and L for examples) agreed with a doctor and the patient.
Criteria for supervising doctor or dentist:
- a registered medical practitioner.
- a specialist registrar, clinical assistant, or consultant, including locums
- a practitioner within a general practitioner (GP) practice
- responsible for the initial assessment and diagnosis on which the CMP is based, and for the preparation of the CMP
- responsible for defining the parameters of prescribing within the CMP
- responsible for providing the necessary support, advice, and supervision to the supplementary prescriber, as required
5.2.2 The supplementary prescriber
The supplementary prescriber will:
- have successfully completed an approved University-based non-medical prescribing course
- have had such qualification registered with their appropriate professional body, for example, NMC, GPhC and HCPC. They will provide evidence of such registration to the head NMP for inclusion on the trust register and database
- prior to the commencement of the post, authorisation for the post to be a supplementary prescriber will be completed and signed by the NMP, the supervising practitioner (SP), care group NMP lead (appendix G) (the NMP lead will link with management colleagues and, or nurse director as appropriate to agree on suitability)
- supply a sample signature, (appendix E), a record of which will be maintained centrally, by the trust’s head NMP, which will be available for checking prescription signatures against
- be responsible for prescribing within the parameters identified in the CMP
5.2.3 The supplementary prescribing process
Before undertaking prescribing, the supplementary prescriber will:
- reach agreement with the care group NMP lead that supplementary prescribing is to be part of their professional responsibilities and that this is suitably reflected within their job description
- ensure arrangements are in place for access to prescription pads, or that other mechanisms for prescribing, appropriate to the clinical setting, are organised (such as FP10s (which are prescriptions that the prescriber uses to write the prescription onto), electronic prescribing or paper-based drug charts)
- have entered into a prescribing partnership agreement with an appropriate independent prescriber(s) (as detailed above) and ensure that this is recorded in the patients’ electronic clinical records
- agree the CMP with the SP, obtain the patients’ agreement to such an arrangement, and that all relevant parties to such an agreement will maintain any patients’ records jointly (preferably within the electronic patient record)
- ensure that all such agreements are always within their clinical competency and their professional code of conduct
- ensure that the CMP is reviewed at agreed intervals and no less frequently than annually
- both the independent and the supplementary prescribers must record their agreement to the continuing or amended CMP, and the patient’s agreement to the continuation of the supplementary prescribing arrangement, for the CMP to remain valid
- ensure that the patient’s general practitioner (GP) is informed of changes to the CMP and to the patient’s medication, in as prompt a manner as is practically possible
5.2.4 The clinical management plan (CMP)
- It is a requirement that any patient for whom supplementary prescribing is to take place, will agree with this arrangement prior to it commencing. The principles underlying this arrangement must, therefore, have been explained to them in advance, and this agreement recorded in the patient’s records.
- In the event of either parties to the CMP ceasing to be involved in the care or treatment of the named patient, the CMP may not continue, until a new CMP has been agreed and put into place.
- The CMPs used will be based upon the examples in the appendices H and 9 (for example, as prepared by the Department of Health (DH)), which represent the minimum acceptable standard for a CMP.
- All sections of the CMP must be completed.
- Following assessment and diagnosis by the independent prescriber, either the independent or supplementary prescriber will draft the CMP after discussion as to its content. Both parties must agree its content before supplementary prescribing can begin.
5.2.5 The clinical management plan comes to an end if
At any time, at the discretion of either prescriber or the patient; at the time specified for the review of the patient (unless it is renewed by both prescribers at that time).
If a new independent prescriber takes over prescribing, the CMP must be reviewed and agreed by the new independent prescriber, the patient and supplementary prescriber.
5.2.6 Medicines prescribed under supplementary prescribing arrangements
The NMP will undertake best practice prescribing in accordance with Department of Health, British National Formulary (BNF), National Institute of Health and Care Excellence (NICE) and RDaSH’s guidelines (including direction from the Medicines Management Committee) and current regulation.
Each NMP will generate a formulary with their SP. This individual formulary will be confined to medicines that the NMP is clinically competent to prescribe and medicines that the NMP will need to be able to prescribe within their role.
The NMP, NMP line manager and SP will determine that a formulary is appropriate. The care group NMP lead will then need to agree to this role for the NMP. This must be agreed prior to the role being put in place.
5.3 Independent prescribing
Independent prescribing is prescribing by a practitioner, who is responsible and accountable for the assessment of patients with undiagnosed or diagnosed conditions and for decisions about the clinical management required, including prescribing.
An independent prescriber can prescribe any medicine within their clinical competence, agreed formulary and job role.
The non-medical prescriber must prescribe from a formulary that:
- has been agreed between the individual clinician and their NMP supervisor
- is agreed and articulated as a BNF section with exclusions, rather than a specific list of drugs
- reflects the service which is being commissioned and delivered and considers the clinician’s competencies
5.3.1 Requirements for supervising practitioners (SP)
The SP can be a registered medical practitioner or appropriate peer NMP (for example, an individual who has undertaken a recognised NMP training course, is an active prescriber, be at least one year post NMP qualification and who is able to meet the supervision needs of the practitioner within their area of practice). The supervisor should have extensive experience as an NMP or prescriber and should offer the supervisee regular supervision on their prescribing practice and record this on the staff portal as NMP supervision. If supervision is sought external to the trust, the practitioner can record this on the portal using the external supervision tab. The supervisor signs off the annual declaration for their supervisee if they are confident in the prescribing practices of the supervisee.
They can:
- be a locum or substantive medical professional working within the NMPs speciality
- be a practitioner within a GP practice or appropriate experienced peer NMP
5.3.2 Requirements for NMP independent prescribers and authorisation
- The NMP must have successfully completed an approved university based NMP course.
- The NMP must have such qualifications registered with the relevant professional regulator (for example, NMC, GPhC, HCPC etc).
- The NMP must provide evidence of registration for inclusion on RDaSH’s register and database of NMPs.
- Where possible the job description for the role should require them to be a NMP.
- The NMP must provide RDaSH’s head NMP a specimen signature, which will be available for checking prescription signatures against.
- Prior approval must be obtained for the post to be a NMP by the care group NMP lead prior to application to the NMP course or an established NMP moving to a new role.
- Each NMP must have a supervising practitioner with experience in the supervisee’s area of practice.
- Prior to completion of the form for authorisation to practice the NMP needs to provide evidence of meeting the requirements to practice.
- Prior to the commencement of the post, authorisation for the post to be an independent prescriber will be completed and signed by the NMP, the SP, the care group NMP lead (appendix H) (the NMP lead will link with management colleagues and, or nurse director as appropriate to agree on suitability).
- The authorisation form must be completed before commencing practice with prescribing as an NMP and the practitioner receive the authorisation form back to them fully signed.
5.3.3 The independent prescribing process
- The NMP role (within care groups) must be a part of that individual NMP’s professional responsibility.
- Arrangements must be in place for the costs of prescriptions issued to be met from an identifiable budget.
- The main clinical record will be identified to detail all prescribing activity.
- The NMP will always work within their clinical competency and professional code of conduct.
- The NMP must only prescribe for the patient if they are currently delivering clinical care to that patient or are part of a multi-disciplinary team (MDT) collectively delivering care to patients within their clinical service.
- The NMP must be satisfied that consent to treatment has been adequately considered and where necessary the patient capacity assessed under the Mental Capacity Act. This will be documented as appropriate. Colleagues should refer to the trust MCA Mental Capacity Act 2005 policy.
- All prescribing by another party, for example, GP, needs to be considered prior to the NMP prescribing.
5.4 Community practitioner nurse prescriber
Community practitioners can prescribe independently of a limited nurse prescribers formulary as identified within the BNF.
5.4.1 The community practitioner nurse prescriber
The community practitioner nurse prescriber will:
- have successfully completed an approved non-medical community prescribing course that gives them the right to prescribe from the nurse prescriber’s formulary
- have had such qualification registered with their appropriate professional body, for example, Nursing and Midwifery Council, provide evidence of such registration to the Head NMP for inclusion on the trust register and database
- prior to the commencement of the post, authorisation for the post to be a community practitioner prescriber will be completed and signed by the NMP, the SP, care group NMP lead (appendix I) (the NMP lead will link with management colleagues and, or nurse director as appropriate to agree on suitability)
- supply a specimen signature (appendix E), a record of which will be maintained centrally, by the trust’s head NMP, which will be available for checking prescription signatures against
5.4.2 The process for community practitioner nurse prescriber
Before undertaking prescribing, the community practitioner nurse prescriber will:
- reach agreement with the care group NMP lead that community Practitioner nurse prescribing is required within the role and where possible be reflected within their job description
- ensure access to prescription pads, or that other mechanisms for prescribing, appropriate to the clinical setting, are organised (such as FP10s, electronic or drug charts including paper-based if appropriate)
- ensure that the patient’s GP is informed of initiation or changes to the patient’s treatment, in as prompt a manner as is practically possible
5.4.3 Documentation for all non-medical prescribers
- The NMP will make an accurate, contemporaneous record of prescribing.
- The date, name of prescriber, preparation prescribed, dose, frequency and total quantity will be documented.
- The proposed duration of treatment will be documented. This will include intended outcomes such as target symptom reduction or target functional level or overall treatment plan.
- Document consent and mental capacity issues in accordance with RDaSH’s policies and procedures as appropriate.
- The NMP will detail, and document proposed future care (including dose titration or alternative preparations) that have been discussed with the patient. The GP and any relevant professional will be informed of prescribing information and changes to treatment (as required). This may be via SystmOne task, letter or email.
- For inpatient prescribing as above, to be included on discharge letter for GP.
- For supplementary prescriber’s medicinal preparations or items to be ingested, it is required that the name of any prescribed item, strength (if any) of the preparation, the dosing schedule and route of administration is prescribed, for example, “paracetamol oral suspension 120mg/5mls, 5mls to be taken every 4 hours by mouth as required for pain, maximum of 20mls in 24 hours”. (See BNF or NPF) is documented.
- For supplementary prescriber’s topical medicinal preparations, the name of the prescribed item, the strength (if any), the quantity to be applied and frequency of application is to be indicated.
- Where hospital outpatients and, or treatment cards are used for prescribing, the supplementary prescriber will write prescriptions which bear the identifier of the independent prescriber.
5.5 Adverse drug reaction reporting
- Any suspected adverse drug reactions must be dealt with appropriately at the time.
- Where appropriate, referrals are to be completed to other healthcare professionals.
- Should a patient experience a suspected reaction to any prescribed, over the counter or herbal medicine, the adverse reaction must be reported via the yellow card scheme and incidents which require action by a practitioner within the trust or any level of harm to the patient must be reported via the trust incident reporting system (using the IR1 form).
- The Medicines and Healthcare products regulatory Agency (MHRA) and Committee on the Safety of Medicines (CSM) encourage the reporting of all suspected adverse drug reactions to newly licensed medicines that are under intensive monitoring (identified by a symbol both on the product information for the drug and in the BNF) and all serious adverse reactions to all other established drugs.
- Reporting can be completed on-line at MHRA website (opens in new window)
5.6 Prescription pads
- The process for registering newly qualified NMPs with the NHS Business Services Authority (NHSBSA) is the responsibility of the chief pharmacist.
- All prescription pads must be treated as “controlled stationery” and are the property of RDaSH.
- In the event of loss or suspected theft of prescription pads or forms the NMP will report this immediately to their line manager and to the police so that the loss can be investigated. A trust incident form on (IR1 form) must be completed. The NHSBSA and counter fraud specialist need to be informed to reduce the risk of fraudulent use.
- A record of prescription pads and their numbers is currently held by the purchasing department.
- Prescription pads will be kept in a locked and secure place (drawer, cupboard or safe) at all times, other than when in transit. When in transit, it is the responsibility of the NMP to ensure suitable security and that pads are never left unattended.
- Under no circumstances must the NMP provide blank prescriptions pre-signed prior to use.
- When it is necessary for the NMP to take a prescription pad home the NMP must ensure the pad is securely stored.
- Prescriptions must be completed in accordance with BNF requirements.
5.6.1 Electronic prescribing
It is the responsibility of the NMP to access the appropriate training before commencement of electronic prescribing. This can be accessed through the pharmacy department.
The colleague’s smart card will need updating to give prescribing permission.
All prescribing and the rationale for its use must be recorded as part of the electronic patient record, utilising the electronic prescribing and medicines administration (ePMA) functionality.
5.7 Legal and clinical liability
The trust holds vicarious liability for NMP’s given authority to prescribe and meeting the agreed criteria in the prescribing role.
NMPs are individually professionally accountable to their regulatory body for this aspect of their practice, as for any other, and must always act in accordance with their code of professional conduct.
5.8 Specialist skills and post registration development (SSPRD)
- NMPs will have locally agreed protected continued professional development time. This should be seen as additional to any study time permitted for the usual purposes of clinical updating.
- The NMP will maintain details of study undertaken within their professional portfolio. This activity will be signed off by both the NMP and their supervising practitioner on the annual declaration (appendix Q) which is submitted to the head NMP by 30th April of each year. Via rdash.non-medical-prescribing@nhs.net.
- NMPs are expected to maintain their level of continuing competence, familiarise themselves with best practices, change and developments, within the management of the conditions for which they prescribe.
5.9 Clinical governance, evaluation and audit
- NMPs must have in place arrangements for regular NMP supervision (a minimum of every 90 days), which appropriately supports their prescribing practice and should meet regularly with their SP).
- All NMP supervision is to be recorded on the supervision portal by the supervising practitioner.
- The competency framework for all prescribers will be used as a tool within supervision and for sharing good practice.
- The annual professional development review (PDR) of someone who is a NMP must include a review of prescribing activity and review of the single competency framework to ensure compliance. If changes are agreed to the NMPs competency and formulary they must complete appendix N along with associated training needs.
- NMPs will be expected to cooperate fully with the development and implementation of any audit or research into any elements of prescribing and the impact on patients within the service.
- NMPs will, in addition, be expected to supply any such information about their prescribing as will be necessary to create prescribing or prescriber profiles for the organisation.
- All NMPs will adhere to the guidance held in this policy. The trust head NMP will ensure all existing NMPs are aware of and have access to this policy.
- It is the responsibility of all NMPs to be aware of the policy and its contents.
5.10 Probity and ethical issues
- All NMPs must prescribe within their registration body’s standards of prescribing practice as outlined in the agreed formulary within RDaSH.
- Where a patient is unable to give their consent to the supplementary prescribing agreement to the NMP arrangements, the NMP cannot proceed with the supplementary process.
- If a patient withdraws consent to treatment, the NMP will discuss with the patient the full implications of this decision and discuss the outcome within the MDT setting.
- Under no circumstances may NMPs accept ‘free samples’ of medicines including creams and products.
- NMPs are likely to find that they are having contact with representatives of the pharmaceutical industry. Care must be taken to ensure that prescribers follow trust guidance and policies concerning this relationship as detailed in the trust conflicts of interest policy. An indication of any such conflict must be made as part of the annual NMP declaration, see appendix Q, with more detailed declaration conforming with the requirements of the conflicts of interest policy.
- NMPs are always accountable for their practice. If circumstances arise where an NMP is using their prescribing qualification in private practice outside the trust, for example, aesthetic procedures then they must declare this on their annual declaration. However, the annual declaration only provides governance for NMP prescribing practice that is outlined in their agreed formulary, within the trust and does not support any private work undertaken externally to the trust.
- If the practitioner undertaken NMP work with another trust or authority as well as with RDaSH they must adhere to those organisations processes and policies for that work. This policy relates only to the work undertaken within their role with RDaSH.
- The NMP is personally responsible for ensuring they have appropriate indemnity insurance, supervision and governance framework in place for any private work undertaken outside the trust.
- This policy is to support safe prescribing practice only within the agreed formulary and job roles and responsibilities with RDaSH.
5.11.1 Cease to prescribe
The process to follow can be found as a flowchart in appendix S.
The trust’s head NMP and care group NMP lead, and practitioner’s line manager must be informed if an NMP ceases to prescribe for any reason, or if they leave the trust’s employment. The trust has the responsibility to ensure that all unused prescription material is retrieved and destroyed by confidential shredding and the serial numbers are recorded on the cease to prescribe documentation. For electronic prescribing the trust has the responsibility to remove the practitioner from the PPA(NHSBSA) list and remove prescribing rights form SystmOne.
The NMP project support officer will advise the purchasing department of the destroyed prescription pad serial numbers for their records and inform the chief pharmacist.
5.11.2 Suspension or termination of prescribing rights
The trust reserves the right to suspend or terminate authorisation of prescribing rights of NMPs for the following reasons:
- during investigation into alleged errors or otherwise unsatisfactory clinical practice related or otherwise to prescribing
- as a consequence of an investigation into unsatisfactory clinical practice related or otherwise to prescribing
- in relation to the circumstances of any unsatisfactory practice, the decision to suspend an NMP’s prescribing rights will be made in partnership by the care group NMP lead, care group nurse director and or care group director, who must have sought clinical advice from the head NMP and medical supervisor, pending investigation
Additional matters that might result in a decision to suspend prescribing rights include:
- failure on the part of the NMP to engage in and report detail of specialist skills and post registration development (SSPRD) for the trust register of NMPs
- failure on the part of the NMP to provide a sample signature for the trust register of NMPs
- failure of the NMP to provide a signed annual declaration of their competence to practice receipt of gifts, sponsorship and fees
- failure of the NMP to complete the annual declaration
In relation to an NMP’s failure to provide detail described as required by the trust register of NMPs, the following actions will be progressed:
- trust head NMP will request detail of outstanding information from the NMP directly, with a copy to the NMPs line manager and, or professional supervisor
- should the NMP continue in failing to provide the detail requested, the trust head NMP will contact the care group NMP lead and the NMP’s care group nurse director to inform them of this failure to address this matter
- the care group nurse director will suspend the right to non-medical prescribing should circumstances mean this is necessary
- any NMP who has not actively used their prescribing skills for one year will have their prescribing status reviewed at personal development review (PDR). If NMP status is no longer deemed to be appropriate to the role the NMP will be informed by the manager and the NMP removed from the active register (using appendix S)
- if their active status is removed the NMP will be informed
5.12 Prescribing resumption or prescribing gaps
In the event an NMP has not prescribed since qualification or, ceased prescribing it may be appropriate to commence a resumption to practice. Upon this occasion the trust must be satisfied that an individual is both competent and capable to prescribe safely prior to any resumption or commencement where a gap of more than one year has occurred. The practitioner will not prescribe until they receive re-authorisation.
In order to address this, the following process must be adhered to:
- the NMP must write to the care group NMP lead, using the approved template found in appendix O, informing them of their wish to resume or commence prescribing. This letter must detail dates of being first qualified and or last date of using prescribing skills, the additional training and revision they intend to carry out that has been individually designed to meet their bespoke needs as decided by them and their supervisor
- the NMP must carry out the planned revision or training
- on completion of the revision or training, appendix P must be completed and signed by the NMP’s SP and care group NMP lead and forwarded to the NMP project support officer for processing
- following authorisation by the head NMP, the NMP project support officer will record colleague member details on the trust NMP database
If a NMP is moving service area, then consideration needs to be given to the above process where appropriate.
Note, in circumstances where a NMP has been subject to period of prescribing suspension because of unsatisfactory clinical practice, a process as described above will need to be followed, with distinct identification of issues and a reflection of any requirement agreed as part of an investigation and subsequent action plan. In such cases, an initial letter must be generated by the NMP’s supervising practitioner, care group NMP lead manager or care group nurse director.
The NMP lead and care group nurse director must endorse a final letter confirming any actions necessary to enable a return to prescribe have been satisfactorily completed and send to the head NMP at rdash.non-medical-prescribing@nhs.net.
5.13 Additional role reimbursement scheme (ARRS)
Under the ARRS scheme the primary care mental health practitioners are employed by the secondary care mental health provider but fully embedded within the primary care network (PCN) via a service agreement. The practitioners will function as an Independent NMP as per this RDaSH NMP policy.
6 Training implications
6.1 Electronic prescribing, NMP’s who are going to use electronic prescribing, to undertake training prior to prescribing in SystmOne
- How often should this be undertaken: Once.
- Length of training: 60 to 120 minutes.
- Delivery method: Combination of video or e-learning.
- Training delivered by whom: Learning and Development team or ESR.
- Where are the records of attendance held: Pharmacy department.
As a trust policy, colleagues can be made aware of its contents by a variety of means such as;
- one to one meetings or supervision
- continuous professional development sessions
- daily email (sent Monday to Friday)
- practice development days
- group supervision
- special meetings
- intranet
- team meetings
- local induction
7 Equality impact assessment screening
To access the equality impact assessment for this policy, please email rdash.equalityanddiversity@nhs.net to request the document.
7.1 Privacy, dignity and respect
The NHS Constitution states that all patients should feel that their privacy and dignity are respected while they are in hospital. High Quality Care for All (2008), Lord Darzi’s review of the NHS, identifies the need to organise care around the individual, “not just clinically but in terms of dignity and respect”.
As a consequence the trust is required to articulate its intent to deliver care with privacy and dignity that treats all service users with respect. Therefore, all procedural documents will be considered, if relevant, to reflect the requirement to treat everyone with privacy, dignity and respect, (when appropriate this should also include how same sex accommodation is provided).
7.1.1 How this will be met
No issues have been identified in relation to this policy.
7.2 Mental Capacity Act (2005)
Central to any aspect of care delivered to adults and young people aged 16 years or over will be the consideration of the individuals’ capacity to participate in the decision-making process. Consequently, no intervention should be carried out without either the individual’s informed consent, or the powers included in a legal framework, or by order of the court.
Therefore, the trust is required to make sure that all staff working with individuals who use our service are familiar with the provisions within the Mental Capacity Act (2005). For this reason all procedural documents will be considered, if relevant to reflect the provisions of the Mental Capacity Act (2005) to ensure that the rights of individual are protected and they are supported to make their own decisions where possible and that any decisions made on their behalf when they lack capacity are made in their best interests and least restrictive of their rights and freedoms.
7.2.1 How this will be met
All individuals involved in the implementation of this policy should do so in accordance with the principles of the Mental Capacity Act (2005).
8 Links to any other associated documents
- Conflicts of interest policy
- Consent to care and treatment policy
- Designated prescribing practitioner competency framework (opens in new window)
- Healthcare record keeping policy
- Prescribing competency framework (opens in new window)
- Safe and secure handling of medicines manual
9 References
- British National Formulary (opens in new window)
- Nurse Prescribers’ Formulary for Community Practitioners (opens in new window)
- Nursing and Midwifery Council (2018) The Code – Professional Standards of Practice and Behaviour of Nurses, Midwives and Nursing Associates (opens in new window)
- Royal Pharmaceutical Society (2016) A Competency Framework for All Prescribers (opens in new window)
- Royal Pharmaceutical Society (2021) A Competency Framework for Designated Prescribing Practitioners (opens in new window)
10 Appendices
10.1 Appendix A Non-medical prescribing training pathway
- Role developed that requires the practitioner to be an NMP within the service. Confirmed by the care group or role developed that requires the practitioner to be a NMP as reflected in the job description.
- Authorisation for NMP Training to be is sought from care group NMP lead.
- NMP Application to be discussed between care group NMP lead, and service manager
- Application and study leave form or DocuSign process completed by colleague and appendix B then sent to care group NMP lead.
- Care group non-medical prescribing lead signs off the NMP application.
- Application is authorised by care group NMP lead, and sent to central email for details added to NMP tracker document rdash.non-medical-prescribing@nhs.net.
- Application is forwarded to the appropriate University by NMP administrator with head of learning and development, colleague and care group NMP lead copied in.
- University place allocated to colleague and further correspondence to be directly with university.
- On completion of course colleague informs Learning and Development administrator and rdash.non-medical-prescribing@nhs.net.
10.2 Appendix B Letter to support request for NMP training
10.3 Appendix C Non-medical prescribing, authorisation process
- Either:
- NMP entering RDaSH from another trust NMP training course completed, and University qualification attained. Professional body registration
- new NMP training course completed, and University qualification attained. Colleague applies for their professional body registration, for example, NMC) and statement of entry provided indicating prescribing status
- Colleague defines formulary with supervising practitioner.
- NMP request pack to be prepared by colleague:
- appendix F, formulary confirmation letter to care group NMP lead
- appendix G, care group NMP lead approval letter (for supplementary prescribers)
- appendix H, care group NMP lead approval letter (for independent prescribers)
- appendix S, care group NMP lead approval letter (for community prescribers)
- appendix E, sample signature evidence of relevant regulatory body registration
- Care group NMP lead to submit full NMP request pack using email, rdash.non-medical-prescribing@nhs.net copying in the NMP colleague for their portfolio.
- NMP project support officer to record details on the trust NMP database and save documents to colleague NMP file.
- NMP project support officer to register NMPs with the NHSBSA for inclusion on the national database.
- Colleague to order prescription pad (if applicable) from purchasing department, appendix J or undertake electronic prescribing training.
- Colleague to have a RA02 form completed by line manager to give access to the SystmOne prescribing rights.
10.4 Appendix D Letter to support request to become active NMP
10.5 Appendix E Sample signature
10.6 Appendix F Formulary authorisation request
10.7 Appendix G Supplementary prescribing
10.8 Appendix H Independent prescribing
10.9 Appendix I Community prescribing
10.10 Appendix J Prescription pad form
10.11 Appendix K Template CMP 1 (bank), for teams that have full co-terminus access to patient records
10.12 Appendix L Template CMP 2 (blank), for teams where the SP does not have co-terminus access to the medical record
10.13 Appendix M Supplementary prescriber to independent prescriber
10.14 Appendix N New role or change in service
10.15 Appendix O Request to resume to prescribe
10.16 Appendix P Resumption to prescribe
10.17 Appendix Q Annual declaration
10.18 Appendix R Non-medical prescribing, ceasing to prescribe process
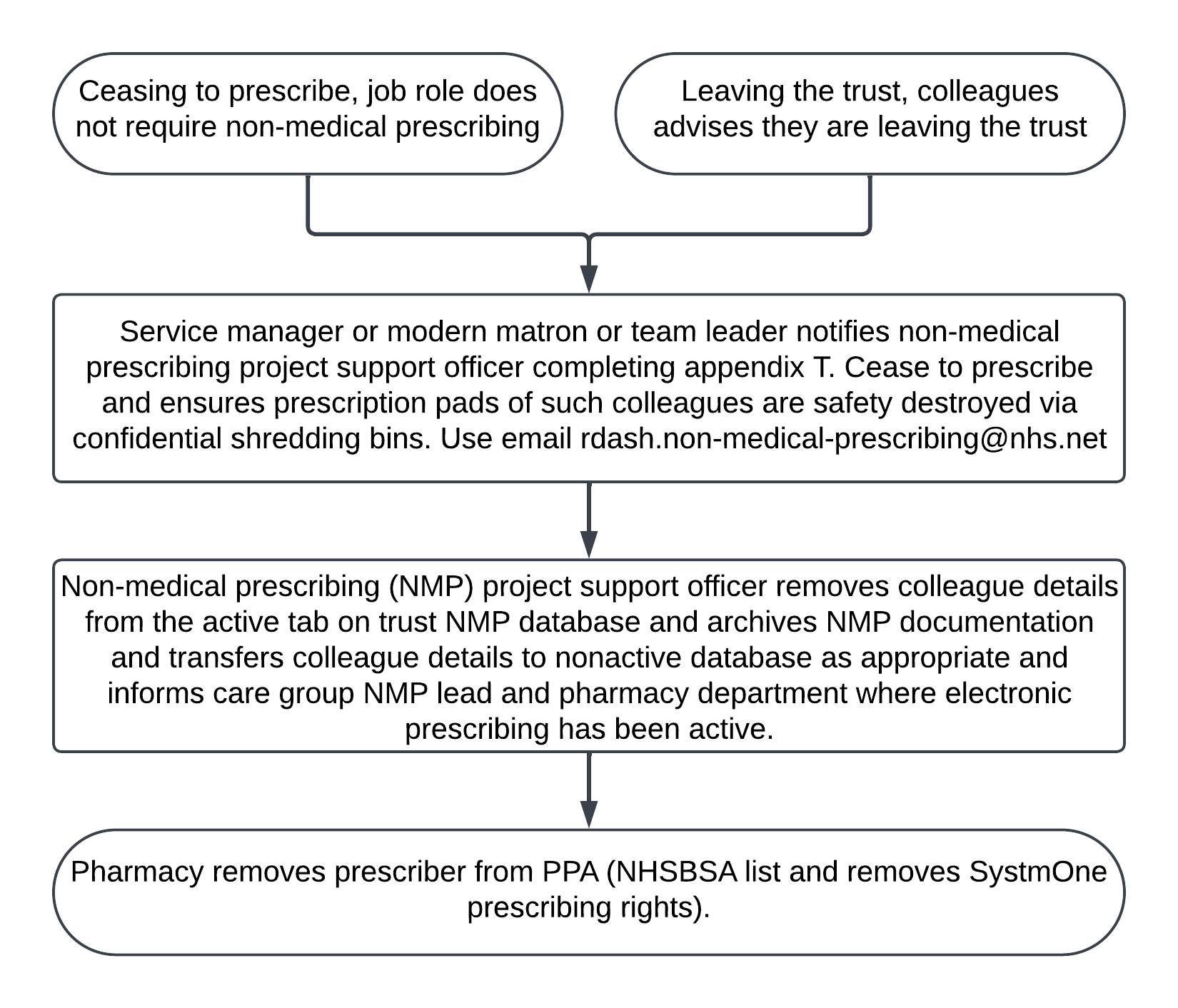
- Either, ceasing to prescribe (job role does not require NMP) or leaving the trust (colleague advises they are leaving the trust).
- Service manager or modern matron or team leader notifies NMP project support officer completing appendix T. Cease to prescribe and ensures prescription pads of such colleagues are safety destroyed via confidential shredding bins. Use email: rdash.non-medical-prescribing@nhs.net.
- NMP project support officer removes colleague details from the active tab on trust NMP database and archives NMP documentation and transfers colleague details to nonactive database as appropriate and informs care group NMP lead and pharmacy department where electronic prescribing has been active.
- Pharmacy removes prescriber from PPA (NHSBSA list and removes SystmOne prescribing rights).
10.19 Appendix S Cease to prescribe
10.20 Appendix T Responsibilities, accountabilities and duties
10.20.1 Chief executive, board of directors, chief operating officer and medical director
The overall responsibility for setting the strategic direction and operational management of the trust policies lies with the chief executive and board of requirements.
The head NMP and medical director are the sponsoring directors for this policy.
10.20.2 Medicines Management Committee
To oversee and support the content of this policy and facilitate prescribing developments through collective multi-disciplinary discussion that then might necessitate ratification of changes to policy.
Note, all proposed operational changes to prescribing practice, be they of a piloting or substantive nature, must be agreed with the head NMP, NMP leads and relevant senior operational managers and the chief pharmacist prior to implementation.
10.20.3 Chief pharmacist is responsible
Chief pharmacist is responsible:
- for giving appropriate support to the head NMP and non-medical prescribing leads
- to ensure non-medical prescribers (NMPs) have access to expert pharmaceutical advice when required
- to oversee the governance of non-medical prescribing to ensure this is appropriate and robust
- to ensure prescribing practice is audited and monitor any prescribing trends that have been identified
- will ensure all NMPs who prescribe via the EPS or FP10 will have prescribing data available
- ensure that arrangements are in place for the costs of prescriptions issued, to be met out of identifiable budgets
- ensure the PPA (NHSBSA) list is up-to-date
10.20.4 Head NMP
- In partnership with NMP leads maintain an up-to-date register of all NMPs (a statutory requirement).
- To support the development and maintenance of specialist skills and post registration development (SSPRD)
- To support recruitment and selection of NMPs.
- To work with the care groups in developing non-medical prescribing.
- Provide advice and support to NMPs.
- Ensure each NMP signs an annual statement of probity.
- Links with the chief pharmacist and disseminating information.
10.20.5 Care group NMP leads and care group nurse directors
Care group NMP leads, directors and care group nurse directors are responsible for:
- providing leadership, governance and support to NMPs in the care group
- supporting workforce development in regard to non-medical prescribing within their care group
- facilitating their colleagues access to and compliance with the procedures described in this document
- implementing agreed training or education methods or programmes to support the procedures described in this document
- establishing mechanisms for regular evaluation of the implementation and effectiveness of this policy. To act as liaison between their care group NMPs and the NMP group
- supporting investigations relating to non-medical prescribing errors and oversee remedial plans within their care groups
- overseeing the NMP process within the care group
- reviewing applications to ensure the applicant meets the criteria, sign for approval and refer on to head NMP for processing (care group NMP lead)
- discussing any inappropriate applications and giving rationale for the decision
- upon successful completion of the course and registration with the NMC, when the request is sent to the care group NMP lead
- supporting and supervise colleagues who have not successfully completed the course to continue to successful completion
10.20.6 Service managers and modern matrons
Service managers and modern matrons are responsible for ensuring this policy is implemented and monitored within their area of responsibility and remain responsible for the support and supervision of their colleagues. They should also:
- provide appropriate advice for the storage and safety of prescription pads
- support NMPs in their clinical practice, maintaining adequate provision of clinical supervision and signpost to appropriate clinicians and supervisors
- ensure that NMPs take appropriate action in the case of lost or stolen prescription pads as described in section 5.6 of this policy
- through PDR ensure that all NMPs are receiving the appropriate NMP supervision
- notify the NMP project support officer of any NMPs who leave the service or cease prescribing as soon as possible in writing, ensuring prescription pads of such colleague are safely destroyed (see appendix S)
- in partnership with the NMP lead annually review job descriptions to determine and identify the needs of the service
- advise practitioners during the application process of non-medical prescribing training to identify who their designated prescribing practitioner (DPP) has agreed to be to support them through the course and direct them to follow the process as outlined at appendix A of this policy
10.20.7 Designated prescribing practitioner (DPP)
Regulatory changes in 2019 meant that experienced non-medical prescribers of any professional background can become responsible for a trainee prescriber’s period of learning in practice as previously undertaken by designated medical practitioners (DMP).
To help train safe and effective independent prescribers, the Royal Pharmaceutical has published the competency framework for designated prescribing practitioners (DPP) (opens in new window).
10.20.8 Non-medical prescribers
NMPs are active throughout the various care groups within the trust and have a wide range of roles and responsibilities. NMPs are responsible and accountable:
- for all aspects of their prescribing decisions, and to their employers and regulatory bodies for their actions or omissions
- to only prescribe those medicines they know are safe and effective for the patient and condition being treated within their sphere of competence
- if an NMP moves to a new clinical specialism their competency needs to be discussed and agreed with their line manager, care group NMP lead, supervising practitioner (SP) and the NMP project support officer to be informed to update the trust database. A sample letter advising the change of specialism and formulary is shown at appendix N
- to remain up-to-date with knowledge and skills to enable competent and safe prescribing
- to maintain and update a competency framework
- all NMPs to receive a minimum of quarterly supervision from their supervising practitioner and for appropriate record keeping being maintained and logged within the trust supervision portal
- to complete an annual declaration by the 30 April each year. This declares that the NMP meets minimum supervision requirements, record specialist skills and post registration development (SSPRD) activity, probity statement and prescribing competence) (appendix Q)
- to advises the NMP project support officer of any personal changes, for example, change of surname to enabling the updating of the national and trust database via the NMP email inbox non-medical-prescribing@nhs.net
- to ensure that they are subscribed to the MHRA medical releases
- GOV.uk medicines and healthcare products regulatory agency (opens in new window)
- for the management and control of their prescription pad in line with NHS Counter Fraud Authority guidance
- memoire for prescribers (staff access only) (opens in new window)
10.20.9 Non-medical prescribing support officer
- Maintain an up-to-date register in conjunction with the care group leads of all NMPs within the trust (a statutory requirement).
- Support the NMP leads in the organisation and co-ordination of the provision of continued professional development for NMPs.
- Support the monitoring and auditing of prescribing practice.
- Support the NMP head and NMP leads within each care group in developing NMP within the trust.
- Ensure each NMP submits an annual declaration record (this is to include a relevant declaration against the conflict of interest to the director of corporate assurance).
- Disseminate information throughout the trust regarding NMP.
- Process the registration of newly qualified community NMPs with the NHS Business Services Authority (NHSBSA) to enable the ordering of prescription pads.
- To work in partnership with the chief pharmacist to ensure the PPA (NHSBSA) list and SystmOne recording rights are maintained.
10.21 Appendix U Monitoring arrangements
10.21.1 Compliance with the policy as set out in this document in relation to NMPs, training and NMP database
- How: Review and update of the non-medical prescriber register.
- Who by: The non-medical prescribing project support officer and care group NMP leads.
- Reported to: Medicines Management Committee.
- Frequency: 6 monthly.
10.21.2 Compliance with the policy as set out in this document in relation to non-medical prescribing, analysis of IR1’s
- How: Ongoing analysis of IR1’s by each care group.
- Who by: Clinical effectiveness meetings or care group led meetings.
- Reported to: Medicines Management Committee and care group quality meetings.
- Frequency: Ongoing or as they arise.
10.21.3 Annual declaration
- How: Via NMP supervision, via completion of NMP annual declaration.
- Who by: NMP supervisor.
- Reported to: Head of NMP.
- Frequency: Yearly.
10.22 Appendix V Glossary of terms used
| Term | Meaning |
|---|---|
| BNF | British National Formulary |
| CMP | Clinical management plan |
| DH | Department of Health |
| DMP | Designated medical practitioner |
| DPP | Designated prescribing practitioner |
| ePMA | Electronic prescribing and medicines administration |
| GP | General practitioner |
| GPhC | The general pharmaceutical council |
| HCPC | Health and care professions council |
| MHRA | Medicines and healthcare products regulatory agency |
| MMC | Medicines Management Committee |
| NHS | National Health Service |
| NHSBSA | NHS Business Services Authority |
| NICE | National Institute of Health and Care Excellence |
| NMC | Nursing and Midwifery Council |
| NMP | Non-medical prescriber |
| POM | Prescription only medicine |
| PPA | Prescription Pricing Authority |
| RPS | Royal Pharmaceutical Society |
| SP | Supervising practitioners |
| SCPHN | Specialist community public health nurses |
| SSPRD | Specialist skills and post registration development |
| YCS | Yellow card scheme, organised by the Medicines and Healthcare Products Regulatory Agency (MHRA) or Committee on the Safety of Medicines (CSM) |
Document control
- Version: 10.1
- Unique reference number: 111.
- Approved by: Medicines Management Committee or CPAG.
- Date approved: 6 August 2024.
- Name of originator or author: Assistant director of nursing.
- Name of responsible individual: Chief nurse.
- Date issued: 28 August 2024.
- Review date: 31 December 2026.
- Target audience: All clinical colleagues that are eligible to qualify as a non-medical prescriber and all non-medical prescribers.
Page last reviewed: November 14, 2024
Next review due: November 14, 2025
Problem with this page?
Please tell us about any problems you have found with this web page.