Contents
1 Introduction
1.1 Defining clinical audit
The universally accepted definition for both national and local clinical audit as defined by the National Institute for Health and Clinical Excellence (NICE) in their “Principles for Best Practice in Clinical Audit” is:
- “a quality improvement process that seeks to improve patient care and outcomes through systematic review of care against explicit criteria and the implementation of change. Aspects of the structure, processes, and outcomes of care are selected and systematically evaluated against explicit criteria. Where indicated, changes are implemented at an individual, team, or service level and further monitoring is used to confirm improvement in healthcare delivery.” NICE, 2002
1.2 The purpose of clinical audit
The purpose of clinical audit is to improve patient care which should be seen as a continuous cycle of improvement:
- identifying which topics to audit
- measuring care delivered against standards and evidence-based practice
- acting on the findings, making improvements and changes
- sustaining improvements, including re-audit where appropriate
Clinical audit:
- needs to be a strategic priority for boards as part of their clinical governance function
- is one of the key compliance tools at a board’s disposal and has an important role within the assurance framework
- is a key element of clinical governance
1.3 Clinical audit as a source of board assurance
The aim of a trust’s clinical audit programme is to ensure that effective clinical audits are planned and undertaken to measure and improve the effectiveness and safety of healthcare across the trust, in addition to providing assurance to the board of directors via the board assurance framework (BAF).
The programme should be aligned to the trust’s individual risks as well as taking account of national priorities. For example, if local complaints or surveys illustrate specific, persistent and, or local concerns, the clinical audit programme can be designed to include the monitoring of standards related to those concerns.
Clinical audit can also be used as a systematic tool to address issues which arise about care and treatment priorities from a strategic rather than a more limited local clinical interest.
Clinical audit is a key element of the trust’s risk management strategy.
1.4 The trust clinical audit framework and programme
The trust clinical audit framework provides the structure for audit activity in the trust.
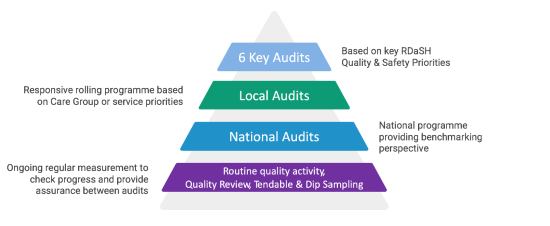
These levels are outlined in more detail below:
- six key audits on fundamental quality of care topics. Focusing on these six priority areas enables the Audit team to provide more in-depth support to care groups, a greater level of analysis and clearer tracking of progress against actions. In turn, services benefit from a consistent approach and greater visibility of quality in these priority areas
- a local, place-based audit programme, each care group will have their own independent audit programme for the year, based on the key practice issues pertinent to them. This programme is intended to be tailored to each care group and dynamic, changing as priorities emerge. For example, if a trust wide audit has demonstrated a good result from an audit, the care group will not be required to repeat that audit in full. However if the outcome of an existing audit was rated as ‘requires improvement’ or ‘inadequate’ the care group will be asked to repeat the audit
- national audits, the trust will contribute to the national audit programme by participating in mandatory audit activity, national audits which are identified as relevant to the services provided by RDaSH. In addition the trust will continue to take part in structured subscription audit programmes such as the prescribing observatory for mental health. Criteria and approaches to these audits are nationally defined
- tendable (formerly known as perfect ward), dip sampling and quality reviews, local quality reviews and other assurance activity will be used to provide a base level of assurance and to identify opportunities for improvement or more targeted measurement using a more formal audit process. Ongoing regular audits will be undertaken using Tendable (formerly known as Perfect Ward) by care groups, supported by facilitated dip samples undertaken locally with support from the audit team or in-house spot checks where appropriate, so the impact of actions taken can be understood, and rapid improvement cycles made possible. The Tendable (formerly known as Perfect Ward) system has a number of audits active, including care records, environmental risks, infection prevention and control, malnutrition universal screening tool, personal protective equipment for COVID-19, rapid tranquilisation, pharmacy and patient experience audits and as these develop results will be tracked to inform the local audit programmes
These reviews, audits and targeted dip samples will also be used to provide additional assurance where issues are identified and to track progress between more formal audits.
The delivery of the clinical audit programme will be planned and prioritised against the available staff resources, with each audit allocated within a specified quarter.
The dynamic local programmes of audit will be approved subject to prioritisation and available resources in consultation with care groups as outlined below.
2 Purpose
The purpose of this policy is to set out the trust’s arrangements and processes for ensuring that all clinical audits are undertaken, completed and reported on in a systematic manner, that is implemented and monitored.
The trust promotes a commitment to the principle of involving service users and carers where appropriate in the clinical audit process as part of its patient engagement strategy.
The trust encourages clinical audit activity undertaken jointly across professions and across organisational boundaries. Partnership working with other local and national organisations will be encouraged where improvement to the patient journey may be identified through shared clinical audit.
3 Scope
This policy applies to all clinical staff that, in the course of their work are expected to contribute to clinical audit as a means of reviewing and improving patient care and their own practice; and, to any non-clinical staff involved in the clinical audit process. This includes staff working directly in clinical services as well as those working in a range of corporate services, including finance and contracting, performance and assurance, human resources and learning and development.
4 Responsibilities, accountabilities and duties
4.1 Board of directors
As trusts are regulated and performance managed against their participation in clinical audit and the findings, the board of directors requires assurance that there is a clinical audit policy in place that meets their strategic objectives.
The board’s role is to ensure that clinical audit is strategic; it happens regularly; is clinically and cost effective; and, is linked to the quality, Innovation, productivity and prevention (QIPP) agenda. The Board will achieve this through the governance structures and ultimately via the report from the quality assurance group.
The board of directors has overall responsibility for the monitoring of clinical quality as a whole within the context of clinical or integrated governance. It has a role in driving quality assurance, compliance, internal audit and ‘closing the loop.’
Clinical audit provides a mechanism through which the quality of clinical care can be measured and improved, and as such has a key relationship to the board assurance framework (BAF).
4.2 Quality committee
The quality committee will regularly review audit activity, reported by the Clinical Effectiveness team in terms of progress against the identified programme, the operation of the function itself and the positive impact on the services delivered.
In turn, the quality committee will report to the board of directors on the level of assurance they have about the clinical audit function.
4.3 Chief executive
The chief executive is responsible for the statutory duty of quality and takes overall responsibility for this policy.
4.4 Executive director of nursing and allied health professionals
The director of nursing and quality is the board lead for clinical audit and is responsible for:
- gaining assurance that clinical audit is relevant, focussed and complete through the results being shared, acted on, reviewed and sustained
- a process for consultation with key stakeholders in the setting of priorities for the trust clinical audit programme, including participation in local and national clinical audits
- assuring that all identified risks are added to the relevant risk register (care group or corporate directorate). Where the risk is initially scored as ‘extreme’ (risk score 15 or more) the risk is reported to the Executive Management team for moderation and acceptance onto the extreme operational risk register, and therefore would require separate reporting on to the relevant committee and board of directors
- the recommendations of clinical audits being actioned, by seeking assurance that improvement in care have been made
This lead role is undertaken in close liaison with the medical director. This will be as part of the overall quality framework and will be reported in the trust’s ‘quality account.’
4.5 Deputy director of safety and quality
Deputy director of safety and quality is responsible for:
- assuring that the process for clinical audit is robust, timely and effective, to enable and sustain improvement and achieve best practice
4.6 Designated nurse director
The care group nurse director is the operational lead for clinical audit, supported by the clinical quality lead and the senior clinical audit facilitator.
The care group nurse director will:
- agree the clinical audit programme in consultation with care groups and other services and present the programme for approval to the quality assurance group
- their role includes the ethical oversight of clinical audit, in consultation with other care group nurse directors and relevant others as necessary
4.7 Head of quality, compliance and assurance
The head of quality, compliance and assurance is responsible for:
- overseeing the overall clinical audit function
- linking clinical audit activity to other aspects of quality and safety
- supporting the clinical effectiveness lead in agreeing the priorities for the programme and local care group activity
4.8 Clinical effectiveness lead
The clinical effectiveness lead is responsible for:
- supporting the care group nurse director with the operational management of the clinical audit programme
- supporting the senior clinical audit facilitator and audit team by implementing the escalation process, to ensure timely completion of audits
- working with the senior clinical audit practitioner and care groups to develop the annual clinical audit programme
- circulating final audit reports to the relevant care groups
- contributing to clinical audit reporting to the quality committee and safety and quality operational group
4.9 Senior clinical audit practitioner
The senior clinical audit practitioner is responsible for:
- planning and prioritising the delivery of the clinical audit Programme against the available staff resource
- ensuring that all clinical audits are measurable against recognised guidelines or criteria, for example, NICE or National or trust priorities
- utilising the healthcare quality improvement partnership (2012) criteria and indicators of best practice in clinical audit as a framework to define the markers or indicators of good quality clinical audit
- making available the clinical audit programme to the care groups or other services
- implementing the prioritised clinical audit programme, scheduling the individual audits and allocating clinical audit facilitator support to each audit topic within the available resource, re-prioritising as required
- checking that all clinical audits are registered and that teams are completing the clinical audit cycle
- ensuring that the clinical audit facilitators adhere to the clinical audit policy and processes that are in place
- maintaining clear records and schedules for re-audits required, allocating resources as above
- assuring that the clinical audit team members provide the results of clinical audits in a timely manner to the care groups or other services for discussion, and action planning
- preparing audit update reports on a monthly basis on the progress and outcomes of the clinical audit programme to the care groups
- managing the request and approval process (including adding additional topics to the approved trust clinical audit programme) from the care groups and other services
- providing clinical audit awareness sessions on request, to clinical teams and individuals
- facilitating and monitoring the dissemination of clinical audit reports and results to a range of identified groups
- following best practice standards when planning, developing, and analysing clinical audit findings and use all clinical audit procedural documentation as outlined in this policy
4.10 Clinical audit facilitator
Clinical audit facilitator is responsible for:
- supporting the senior clinical audit practitioner in the delivery of the responsibilities set out above
- taking the lead role on identified clinical audits with responsibility for implementing and managing the audit process through to completion within agreed timescales ensuring accurate timelines are completed within each clinical audit report
- overseeing the collection of data, completes data analysis, interprets the findings and produces the audit report
- providing support to identified clinical audit leads for any additional audit activity not already included on the clinical audit programme. Assisting them with implementing and managing the audit process through to completion within the agreed timescales
- providing guidance or advice on clinical audit and quality improvement methods and practices
- providing clinical audit awareness sessions on request to clinical teams and individuals subject to audit staff resources
- escalating any urgent risks identified at any point during the audit process immediately to the relevant person(s)
- attend audit meetings with the audit champions for their designated care group(s) to discuss audit activity and escalate any exception reporting to the care group quality meetings
- maintain regular contact with audit champions to progress the audit activity
4.11 Care group nurse directors or care group medical directors and senior managers or clinical leads in other services
Responsibilities:
- promoting clinical audit within their services as a key element of clinical governance; as a quality improvement process that seeks to improve patient care; and, as a continuing professional development (CPD) activity
- service development and delivery being underpinned by clinical audit
- identifying clinical audit priorities and feeding these through to the clinical audit programme, whilst ensuring that these are measurable against recognised guidelines or criteria, for example, NICE or National or trust priorities
- ensuring that all clinical audit activity within their care group is registered centrally through the Clinical Audit team, regardless of whether support from the team is required
- allocating staff resources to undertake clinical audit
- fully considering and acting on the results of clinical audit, feeding identified risks through to the risk register and completing action plans and re-audits as required to make improvements
- ensuring that any additional clinical audits not included on the work programme have a clinical audit work request and registration Form submitted to the Clinical Audit team (appendix A) and follow the submission of clinical audit work request form process (appendix B)
4.12 Service lead or managers
Managers of clinical services are responsible for:
- participating in and engaging their staff in clinical audit as a key quality improvement activity and as part of their CPD
- including clinical audit activity in the objectives of individual staff as part of their annual performance and development review (PDR) process and any identified learning and development needs reflected in their personal development plan
- utilising the clinical audit programme as the source of clinical audit topics for all staff and trainees undertaking clinical audit activity
- disseminating clinical audit results within their teams and using them to drive local quality improvement strategies
- completing, reporting and producing evidence on clinical audit action plans to the Clinical Audit team
4.13 Clinical audit lead
Are responsible for:
- ensuring that audits are being undertaken against clear guidelines or criteria, for example, NICE or national or trust priorities
- discussing and agreeing on a viable sample size and methodology, to support reliable and robust clinical audit results
- being involved in the development of the audit questions or criteria before any data collection takes place to ensure questions are valid, objective and based on agreed standards
- supporting the care groups and clinical services to develop action plans ensuring that SMART (specific, measurable, achievable, realistic and timely) principles are utilised
- following guidance from the Clinical Audit team ensuring action plans are designed to deliver the recommended quality improvements
- keeping the service lead(s) updated on progress and to jointly agree the recommendations and action plan and feedback to the clinical audit facilitator
- sharing results initially with the care group director or care group nurse directors or medical director or lead consultant
- clearly identifying (including name and title) individuals responsible for implementing or closing assigned actions, for audit team monitoring purposes
- ensuring that the required communication has been undertaken to support the identification of appropriate named action leads and that consequently they are aware of this prior to the action plan being distributed or shared
- ensuring that the clinical audit work request and registration forms are fully completed for any audits not included in the annual clinical audit programme
- assuring that they (or a deputy) attend relevant meetings as requested to feedback on their report for example, care group quality meetings
- keeping colleagues in the service up to date with results, issues and quality improvements as the audit progresses
- working in collaboration with the clinical audit facilitator to provide confirmation that the final report is agreed
- undertake action plan reviews with the clinical audit facilitator as appropriate
4.14 Clinical audit champions
Are responsible for:
- raising the profile of clinical audit within their care groups
- supporting audit leads and others within care groups to effectively contribute to clinical audit activity
- acting as a communication link between care groups and the Clinical Audit team
- meeting and maintaining regular contact with the clinical audit facilitator
- follow-up on audit activity or actions that are due or overdue to allow progression of the audit with the audit lead and or care group quality meetings
- exception reporting to the care group quality meetings
- provide feedback to the clinical audit facilitator following the care groups quality meeting
4.15 Safety and quality operational group
The safety and quality operational group (SQOG) assures that there is an appropriate process in place to monitor and ensure compliance with clinical standards and guidelines, including, but not limited to clinical audit. SQOG will provide assurance on the high standards of care delivered by the trust across the three domains of quality: clinical effectiveness, patient safety and patient experience. This group is authorised by the board of directors to act within its terms of reference and ensure all statutory elements of clinical governance are adhered to and will receive summary reports on all completed audits.
The group is responsible for overseeing the trust’s clinical audit activities and will:
- receive reports, quarterly and by exception on the progress and outcomes of the clinical audit programme
- contribute to, receive and approve the clinical audit programme, including the identified key audits, ensuring it is consistent with the quality improvement priorities of the trust
- ensure there is an appropriate mechanism in place for action to be taken in response to the results of clinical audit and the recommendations of any relevant external reports
4.16 Care group quality meetings
The clinical quality group is responsible for:
- delegated responsibility from the SQOG for the process of monitoring clinical audit
- receiving and reviewing proposals for clinical audits for inclusion onto the clinical audit programme
- monitoring progress with clinical audit action plans
- acting as a forum for sharing the results of clinical audits and any related actions and recommendations
4.17 Other trust groups
As well as the formal progress reports provided to the safety and quality operational group, clinical audit outcomes and learning will be discussed where appropriate by:
- medicines management committee
- mortality surveillance group
- environmental risks in clinical areas group
- safeguarding assurance group
- pressure ulcer harm reduction group
- learning disabilities quality circle
- patient experience steering group
- operational management meeting
- mental health legislation operational group
5 Procedure or implementation
5.1 Process for setting priorities for the clinical audit programme, including participation in local and national clinical audits and approved process for audit
This will include discussions at or with:
- the safety and quality operational group where each care group is represented
- care group or other meetings and via e-mail
- corporate level with relevant directors
- commissioners
The safety and quality operational group will agree the clinical audit programme and recommend it for approval to the quality Committee.
The programme will be responsive to identified risks, care group or other priorities, internally and externally.
New topics may be added to the clinical audit programme following receipt of the clinical audit work request and registration form. These will be taken to the care group quality meetings for discussion where a decision will be made whether it will be added to the clinical audit programme or not depending on priorities of the trust and staff resources in clinical audit.
A record will be maintained by the Clinical Audit team of clinical audit proposals which are not approved, and the reasons fed back to the proposer.
The clinical audit programme will provide a source of topics for staff and trainees wishing to participate in audit activity and is shared with junior doctors at their induction to the trust via email or paper copy of the programme.
Identified care group meetings may be utilised to provide a key forum where staff from the range of professional groups can come together to discuss, present and debate clinical audit findings.
5.2 Process for ensuring appropriate standards of performance are audited
Clinical audits will be designed and agreed with care groups, or other services by the Clinical Audit team in order to ensure that quality standards are selected, or new standards are developed which meet trust or care group priorities.
5.3 Involving medical students and junior doctors
Medical students and junior doctors will be encouraged to be involved in clinical audit wherever possible as part of their learning and development. Their audits will be monitored by their supervising Consultant and follow the same process as all other audits.
5.4 Involving patients and carers
Patients and carers often assess quality of care in different ways to healthcare professionals; can provide a unique perspective based on their personal experience; and, can help design services around service user needs.
Involvement may range from passive input, for example, the trust may decide to audit an issue highlighted by complaints, to active engagement, for example, direct involvement of patients in any or all aspects of the clinical audit process.
5.5 Process for disseminating clinical audit results or reports
Draft audit reports will be sent out by the clinical audit facilitator to the audit lead (service or care group) to be placed on the agenda of their care group meetings for presentation, discussion and action planning.
Each report contains a distribution list and the reports are disseminated via e-mail by the clinical effectiveness lead to each of the individuals identified on this list once the report and action plan has been approved by the care group quality meeting. Please see below for generic distribution list:
- head of quality, compliance and assurance
- care group director
- care group nurse director
- care group medical director
- chief pharmacist
- clinical effectiveness lead
- audit lead
- any named staff as lead for audit actions
5.6 Format for all clinical audit reports for example, methodology, conclusions, action plans etc
A streamlined user-friendly audit report format is utilised (appendix C).
The overall rating for the audit will be determined by the audit facilitators and rated as one of the following (CQC judgement framework):
| Outcome rating | Rationale |
|---|---|
| Outstanding | 100% all standards |
| Good | 75% to 99.9% standards |
| Requires improvement | 50% to 74.9% most standards or mixed results |
| Inadequate | 49.9 or below for most standards |
The outcome rating is calculated by working out the overall percentage achieved taking into account the numerators and denominators for each criterion.
It should be noted that there may be occasions when the rating may be altered if any criteria is rated Red and when utilising clinical judgement is deemed to be a patient or staff risk.
For example, if the overall rating is good it may mean that the rating will be changed to require improvement where the risk is seen as high and will automatically become a re-audit. This decision will be made by the care group quality meeting where the clinical audit lead is presenting their report.
Audit reports will evidence improvements in patient care through the development and measurement of evidence‐based practice supported by ‘SMART’ actions:
- specific
- achievable
- timely
- measurable
- realistic
All audits undertaken will have a standard approach. Mechanisms will be in place to support the monitoring of achievements via existing meeting structures (for example, care group quality meeting and confirm and challenge (by peers) of audit actions to support improvement in practice within stated timescales. This will ensure that the audit action plans lead to change in practice within the stated timescales. A change status key is incorporated within the report for audit actions to determine the progress and status upon reporting:
- recommendation agreed but not yet actioned
- action in progress
- recommendation fully implemented
- recommendation never actioned (state reasons)
- other (provide supporting information)
A format which is to be used for all clinical audit reports is provided in appendix C.
The use of this format will promote consistent and comprehensive reporting and will support reporting on the outcomes of the clinical audit programme trust wide.
5.7 Process for making improvements
Clinical audit results will be used to inform improvements where the need is identified.
The action plan will state what action is required against the criteria, the evidence required, by whom and when the action will be completed.
Action plans are monitored to completion by the Clinical Effectiveness team feeding into the care group quality meetings and learning shared within the group.
5.8 Process for monitoring action plans and carrying out re audits
All action plans are entered onto the clinical audit department’s action plan log by the Clinical Effectiveness team.
Due dates for the actions are clearly recorded and the status of the action is recorded.
The spread sheet is reviewed monthly by the clinical effectiveness support officer for any “due” actions.
Any actions which are “due” are followed up by the process below:
- the clinical effectiveness support officer will email the relevant lead(s) responsible for completion, asking them to confirm if the action has been completed and to provide evidence of what has been done in response to the action. A response time of 2 weeks is allocated
- if no response is received the clinical effectiveness support officer will email the relevant lead(s) responsible for completion 1 week after the due date. A response time of 1 week is allocated. The relevant lead(s) responsible for completion will be informed that further monitoring will be carried out by the clinical audit facilitator via the care group quality assurance meetings
- the clinical audit Facilitator will escalate the actions to the relevant care group at the audit pre-meetings with the care group champions.
- the audit champions will escalate any overdue actions to the care group quality assurance meetings
- after the action has been taken to 2 care group quality assurance meetings with no resolution appropriate it is escalated in the first instance to the senior clinical audit practitioner who will escalate this to the clinical effectiveness lead
- the clinical effectiveness lead will contact the line manager of the relevant lead(s) responsible for completion primarily with a 1-week deadline for response
- if no response the clinical effectiveness lead will escalate to the relevant next person in the management structure with a 1-week deadline for response
The action plan log is updated with the comments received, and the status revised accordingly.
Audits are monitored through the clinical audit work programme by the senior clinical audit practitioner and re-audits are scheduled into the clinical audit work programme following the outcome of the action plan review.
The action plan log is monitored by care group quality meetings on a monthly basis.
The results of all clinical audits will be risk rated by the relevant care group and added to the risk register as required by the care group nurse director. These will then be worked through via an action plan by the relevant care group. Director to mitigate the risks. Monthly reviews of risk registers are undertaken by each care group.
5.9 Route for escalation of concerns
The senior clinical audit practitioner will be the first point of contact for the escalation of concerns, raised by the Clinical Audit team. These concerns may be about the clinical audit process, findings or any other related issues.
The senior clinical audit practitioner will in turn discuss concerns with the clinical effectiveness lead as appropriate and follow up accordingly.
In the event of the concerns relating to poor compliance with data submission deadlines, an e-mail will be sent to the relevant service manager with the care group nurse director copied in. This e-mail will provide a final date for data submission and make it clear that if the data is not provided to the audit department by that date a non-submission will be documented in the final audit report.
In the event of the concerns relating to non-completion of action Plans within the audit reports, the clinical audit facilitator will escalate this in the first instance to the senior clinical audit practitioner who will, if necessary, then escalate to the clinical effectiveness lead. If no response is received the matter will then be taken to the care group quality meeting for a decision to be made on the way forward with the audit.
5.10 Action plan reviews
- Following an audit or re-audit an action plan review will be carried out depending on the audit or re-audit outcome:
- inadequate, unannounced after 1 month
- requires improvement, unannounced after 3 months
- good, announced after 6 months
- outstanding, self-audit within 12 months (carried out by the service)
- The senior clinical audit practitioner will contact the audit lead informing them that the review is due to take place.
- For unannounced visits the clinical audit facilitator will select a day for the review and visit the service without warning, or spend time with the audit lead looking at the actions and records.
- For announced visits the clinical audit facilitator will arrange a suitable date with the audit lead.
- The action plan review will be carried out by the clinical audit facilitator and the audit lead and, or other relevant parties.
- The action plan review will be carried out face-to-face or in an MS Teams call.
- The action plan review will look at whether actions have been completed and whether these have been effective by examining a sample of records against criteria achieving inadequate or requires improvement.
- Where the review does not demonstrate improvements, further actions will be put in place.
- If actions had not been completed or there was a need for additional actions at the first review a further action plan review will take place.
- The action plan review will inform any re-audit plans.
5.11 Clinical audit project update
The clinical audit project update spread sheet is an internal resource of the Clinical Audit team and holds details of all clinical audits for monitoring and assurance purposes.
The Clinical Audit team uses a spread sheet to record and monitor the current status of all clinical audit work in progress.
The spread sheet is used by the Clinical Audit team to retrieve appropriate information about projects to support the evidencing and reporting of work and its progress.
6 Training implications
There are no specific training needs in relation to this policy, but clinical staff will need to be familiar with its contents and any other individual or group with a responsibility for implementing the contents of this policy.
As a trust policy, all staff need to be aware of the key points that the policy covers. Staff can be made aware through a variety of means such as:
- trust wide mail drop
- team meetings
- one to one meetings or supervision
- posters
- CPD sessions
- trust wide email
- group supervision
- practice development days
- local induction
The clinical audit team will hold 1-to-1 and group awareness sessions on request to clinical teams and individuals.
7 Monitoring arrangements
7.1 How the organisation sets priorities for clinical audit including local and national requirements
- How:
- clinical audit programme
- report progress
- Who by: Head of quality, compliance and assurance, clinical effectiveness lead and the senior clinical audit facilitator.
- Reported to: Safety and quality operational group.
- Frequency:
- annual
- quarterly
7.2 Requirement that audits are conducted in line with the approved process for audit
- How: Ongoing monitoring of clinical audit reports, new work requests and action plans.
- Who by: Senior clinical audit practitioner.
- Reported to: Care group quality meetings.
- Frequency: Monthly.
7.3 How audit reports are shared
- How: Ongoing monitoring of clinical audit reports, new work requests and action plans.
- Who by: Senior clinical audit practitioner.
- Reported to: Care group quality meetings.
- Frequency: Monthly.
7.4 Format for all audit reports, including methodology, conclusions, action plans etc
- How: Ongoing monitoring of clinical audit reports, new work requests and action plans.
- Who by: Senior clinical audit practitioner.
- Reported to: Care group quality meetings.
- Frequency: Monthly.
7.5 How the organisation makes improvements
- How: Ongoing monitoring of clinical audit reports, new work requests and action plans.
- Who by: Senior clinical audit practitioner.
- Reported to: Care group quality meetings.
- Frequency: Monthly.
7.6 How the organisation monitors action plans and carries out re-audits
- How: Action plan log.
- Who by:
- senior clinical audit practitioner
- head of quality, compliance and assurance or clinical effectiveness lead
- Reported to:
- care group quality meetings
- safety and quality operational group
- Frequency:
- monthly
- quarterly
8 Equality impact assessment screening
To access the equality impact assessment for this policy, please email rdash.equalityanddiversity@nhs.net to request the document.
8.1 Privacy, dignity and respect
The NHS Constitution states that all patients should feel that their privacy and dignity are respected while they are in hospital. High Quality Care for All (2008), Lord Darzi’s review of the NHS, identifies the need to organise care around the individual, ‘not just clinically but in terms of dignity and respect’.
As a consequence the trust is required to articulate its intent to deliver care with privacy and dignity that treats all service users with respect. Therefore, all procedural documents will be considered, if relevant, to reflect the requirement to treat everyone with privacy, dignity and respect, (when appropriate this should also include how same sex accommodation is provided).
8.1.1 How this will be met
No issues have been identified in relation to this policy.
8.2 Mental Capacity Act
Central to any aspect of care delivered to adults and young people aged 16 years or over will be the consideration of the individuals’ capacity to participate in the decision-making process. Consequently, no intervention should be carried out without either the individual’s informed consent, or the powers included in a legal framework, or by order of the court.
Therefore, the trust is required to make sure that all staff working with individuals who use our service are familiar with the provisions within the Mental Capacity Act (2005). For this reason all procedural documents will be considered, if relevant to reflect the provisions of the Mental Capacity Act (2005) to ensure that the rights of individual are protected and they are supported to make their own decisions where possible and that any decisions made on their behalf when they lack capacity are made in their best interests and least restrictive of their rights and freedoms.
8.2.1 How this will be met
No issues have been identified in relation to this policy.
9 Links to any other associated documents
- RDaSH intranet clinical audit page
10 References
- NICE (2002) Principles for Best Practice in Clinical Audit
- Healthcare Quality Improvement Partnership (2010) Clinical Audit: A simple guide for NHS Boards and Partners
- Healthcare Quality Improvement Partnership (2012) Criteria and indicators of best practice in clinical audit
11 Appendices
11.1 Appendix A Clinical audit work request
11.2 Appendix B Submission of clinical audit work request and registration form to the clinical audit team (CAT)
11.3 Appendix C Clinical audit report template
Document control
- Version: 7.3.
- Unique reference number: 367.
- Approved by: Clinical policy review and approval group.
- Date approved: 12 November 2024.
- Name of originator or author: Head of quality, compliance and assurance.
- Name of responsible individual: Chief nurse.
- Date issued: 20 November 2024.
- Review date: 31 July 2025.
- Target audience: All staff who in their course of their work undertake duties in relation to clinical audit.
Page last reviewed: November 20, 2024
Next review due: November 20, 2025
Problem with this page?
Please tell us about any problems you have found with this web page.